Fig. 5.1
Patient positioning for left-sided pyeloplasty with bed turned towards the robot
For example, when doing left- or right-sided pyeloplasties, we keep the three robotic components in roughly the same floor position and rotate the bed 180°. Initially this created some consternation among the anesthesia team as the head of the patient was now away from the ventilator and anesthesia station. This apprehension was alleviated through dry lab drills simulating this orientation. We found that the bed rotation approach allowed our staff to prep and drape the robot in advance of the patient entering the room.
Learning Curve
As with any new technique or approach, there is an inherent learning curve [8, 9]. Taking steps to accelerate learning curves through identification of early champions; managing expectations; creating preliminary milestones; being forthright with your patients, your nursing staff, and your administration; and understanding the realities of what others have shown with regard to the robotics learning curve will facilitate success in your robotics program.
Subspecialty Participation
Pediatric urologists will most likely be the largest adopters of robotic technology in your institution followed by general surgery. We believe that starting with these two surgical disciplines will yield the fastest and safest ramp-up in the program. Cardiac and otolaryngologic pediatric surgeries are now starting to utilize robotics [10, 11], but unless your institution has a member from one of these specialties with existing sound robotic experience, we recommend starting with urology and general surgery teams. There is also ample crossover among the nursing and scrub technician staff between urology and general surgery as our equipment needs, cavity of approach, and target organs are frequently identical. Once your institution has identified one or two starting services, we recommend identifying clinicians who have a strong background in conventional laparoscopy [5]. These champions tend to be more familiar with laparoscopic access, approach, and equipment which are analogous to robotics. There are many examples of surgeons who have become quite facile in robotics with minimal conventional laparoscopic experience [12, 13], and ultimately expanding the ability of all providers to offer the robotic approach is ideal, but minimizing as many aspects of robotic adoption that may be foreign to the starting roboticist is critical for success. It helps to have at least 2 providers in the program at initiation so that (1) communication with your administration and operating room teams can be defrayed and (2) so that idea sharing is possible to accelerate learning and innovation.
Expectations and Milestones
For the classically trained surgeon, the challenge of standard laparoscopy is often overwhelming, whereas transferring the surgical skills in the robotic environment is easier. Laparoscopically naïve surgeons need between 20 and 25 cases to show proficiency [10]. This has also been seen in other works such as a report by Patel et al. that showed a similar learning curve [13]. Unless the surgeon starting a new program is already experienced, there needs to be proper training. This can be accomplished by visiting an already experienced surgeon at their home institution to observe cases. Expert mentoring is also crucial during your first run of procedures to ensure that you are executing all the key maneuvers. Continual video critiquing of your surgical cases is paramount to fine-tune your skills. It becomes most effective when you watch your recorded cases with a colleague who has the same interests as you in robotic surgery.
Following complete training, patient selection is paramount especially early on in program development. Age, anatomy, body mass index, comorbidities, and previous experience with a surgical procedure either in an open or laparoscopic model need to be carefully picked at the beginning of the surgical experience.
Patient Counseling
In our experience, many families are excited about the option of a robotic approach for their children. Honesty is important to help manage expectations when initiating your practice. It will be predictable that despite as much dry lab training and proctoring you receive, in the beginning, your operative times will take longer than your open or even laparoscopic times. In addition, you are not the only ones in the room on his/her learning curve. Your ancillary staff and anesthesia team are also learning, and inconsistency in the teams will amplify operating room times. Tell your patients that you are initiating your robotics practice, and tell your patients if they are one of the first patients in your fledgling experience. Let them decide if they prefer this. We have found that many patients were excited to be the “firsts,” while other families were more apprehensive. Giving the families information about how your outcomes compare to the literature sends a strong message about your integrity and your appreciation for the trust that the families place in you.
Learning Curve Tracking
In early reports of incorporating robotic surgery into one’s practice, outcomes, fortunately, have tracked the open approaches [14]. Sorenson et al. analyzed their first 33 consecutive robotic pyeloplasties among two pediatric urologists and found that length of stay, postoperative pain scores, and surgical outcomes at a modest follow-up (median 16 months) were analogous between open and robotic approaches. Robotic operative times were consistently longer until a certain threshold of cases (15–20) was approached, whereby operative times fell within 1 SD of the matched open cohort. The majority of this time drop (70 %) was appreciated in the surgical time defined as incision to close. This appraisal showed that the surgeon with a more rapid case volume experience saw a faster drop in his operative times. Complications were clumped towards the initial ten cases and were mostly technical in nature. This study also highlighted the importance of optimal patient selection, a principle not well adhered to by these surgeons. The longest case in the study was a robotic pyelolithotomy and pyeloplasty within the first eight cases of one of the surgeon’s overall robotic experience. This study was limited in that it compared the early stages of experience in the robotic approach to the experience of surgeons who had performed the open approach for decades. This is the challenge with appraising comparative effectiveness data because there is virtually no data on the learning curve of open pyeloplasties.
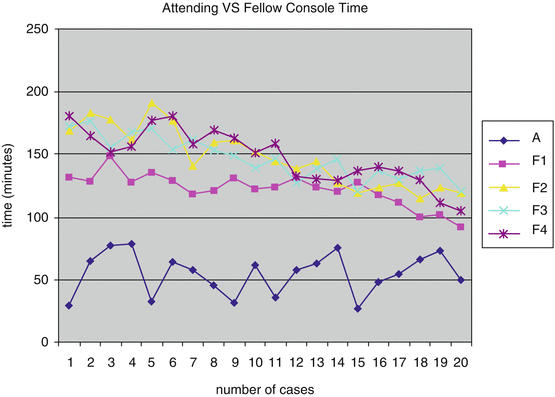
Tasian et al. collected the surgical console times in 20 consecutive robotic pyeloplasty cases of four pediatric urology fellows when they performed 75 % or more of the console time [15]. The console times were compared to 20 consecutive robotic pyeloplasty cases where the attending alone performed 100 % of the console time. All times were validated post procedure by viewing the surgical video and confirming times of console switching. They only evaluated console time. Positioning, prepping and draping the patient, obtaining laparoscopic access, and wound closure were excluded due to participation of other team members. They found the mean console time for the attending operating alone was 54 min. The operative times for the cases in which the fellow performed 75 % of the case decreased with increasing number of cases done (Fig. 5.2).