Jaundice, Newborn
Clyde J. Wright
Michael A. Posencheg
INTRODUCTION
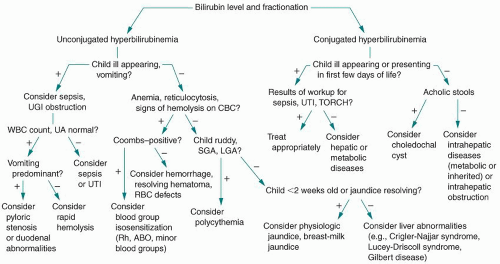
Jaundice is a term used to describe the visible manifestation of elevated serum concentrations of bilirubin. The breakdown products of heme proteins, the most abundant of which is hemoglobin, are the major source of bilirubin. The heme moiety is broken down to biliverdin by heme oxygenase and reduced to unconjugated bilirubin via the action of biliverdin reductase. Unconjugated bilirubin travels to the liver through the bloodstream, mostly bound to albumin, where it is transformed into the conjugated form. Conjugated bilirubin enters the small intestine and is excreted in the stool. Enzymes in the small intestine convert a portion of this conjugated bilirubin back into unconjugated bilirubin, which can return to the bloodstream. Here, it can reenter the hepatic circulation and add to the bilirubin load. This process is called the enterohepatic circulation of bilirubin. Note that elevations of either conjugated or unconjugated bilirubin can cause jaundice, and each etiology has a distinct laboratory evaluation and treatment. For unconjugated hyperbilirubinemia, elevations may be caused by increased production, decreased conjugation or excretion, or increased enterohepatic circulation of bilirubin. Alterations in conjugated bilirubin are primarily caused by bile duct/excretion abnormalities or hepatic dysfunction. Figure 46-1 represents a basic schematic for the consideration of the broad differential diagnosis of hyperbilirubinemia.
Unconjugated Hyperbilirubinemia: Physiologic
Increased Bilirubin Production
Blood group incompatibility—Rh, ABO, and minor blood group
Red blood cell (RBC) enzyme abnormalities—glucose-6-phosphate dehydrogenase (G6PD), pyruvate kinase, and hexokinase deficiency
RBC membrane defects—hereditary spherocytosis, elliptocytosis, and pyknocytosis
Hemoglobinopathies—α-thalassemia, and sickle cell disease
Increased RBC load—cephalohematoma, polycythemia, and ecchymosis
Infants of diabetic mothers
Decreased Bilirubin Conjugation or Excretion
Hormonal deficiency—hypothyroidism and panhypopituitarism
Bilirubin metabolism disorders—Gilbert syndrome, Crigler -Najjar (types 1 and 2), and Lucey-Driscoll syndrome
Sepsis—bacterial, viral, and fungal
Increased Enterohepatic Circulation
Breast-feeding failure jaundice
Breast-milk jaundice
Bowel obstruction or ileus
Pyloric stenosis
Conjugated Hyperbilirubinemia
The differential diagnosis of conjugated hyperbilirubinemia is an extensive list, encompassing more than a hundred distinct disease entities. The majority of the disorders causing conjugated hyperbilirubinemia in the neonates can be organized into obstructive, infectious, metabolic, and miscellaneous categories. Some of the most important etiologies are listed here.
Bile Duct Abnormalities
Biliary atresia
Choledochal cyst
Infection
Hepatic—toxoplasmosis, rubella, cytomegalovirus, herpes simplex (TORCH) infections, and enterovirus
Systemic—bacterial sepsis and urinary tract infection
Metabolic/Genetic Disorders
Galactosemia
Tyrosinemia
α1-Antitrypsin deficiency
Alagille syndrome
Miscellaneous
Total parenteral nutrition (TPN)-related cholestasis
Neonatal hemochromatosis
Idiopathic neonatal hepatitis
SELECTED DIFFERENTIAL DIAGNOSIS DISCUSSION
Unconjugated Hyperbilirubinemia
Physiologic Jaundice
In utero, fetal unconjugated bilirubin easily crosses the placenta and is conjugated and excreted by the mother. Following birth, the neonate is solely responsible and somewhat ill-prepared for bilirubin metabolism. Because of this, jaundice develops in >50% of infants in the first few days of life. Physiologic jaundice never appears in the first 24 hours, and peaks at 4 days in term infants. Many physiologic factors contribute to this “physiologic hyperbilirubinemia,” including a larger RBC mass, decreased RBC life span (70 to 90 days in neonates vs. 120 days in adults), reduced bilirubin uridine diphosphate-glucuronosyltransferase (UDPGT) activity (>1% that of adults in the first 10 days of life), and delayed meconium passage leading to increased enterohepatic circulation.
It is important to note that breast-fed infants have higher bilirubin levels, and with more neonates breast-feeding, clinicians have to reassess what “normal” levels of total serum bilirubin (TSB) are in these otherwise healthy neonates. Historically, 95% of term infants had bilirubin levels that did not exceed 12.9 mg/dL. However, recent studies have shown that in breast-fed infants, the normal mean peak TSB level is 8 to 9 mg/dL, with an upper limit of 17 to 18 mg/dL.
Increased Bilirubin Production
Blood Group Incompatibility
If a blood group incompatibility exists between the fetus and the mother, maternal IgG against fetal red cells can cross the placenta, resulting in hemolysis and increased bilirubin production in the fetus and newborn. An incompatibility can exist in the Rh, ABO, or minor blood group antigens.
Rh Incompatibility
Rh incompatibility occurs when an Rh-negative mother carries an Rh-positive fetus. The mother must have had previous sensitization to the Rh antigen, usually via a prior pregnancy, which results in the production of antibodies directed against the Rh antigen. Rh-positive infants born to Rh-negative mothers display a wide spectrum of disease, ranging from unaffected (15% to 20%) to severe disease, including erythroblastosis fetalis, and fetal death (25%). The administration of blocking antibodies (RhoGAM) to pregnant Rh-negative mothers prevents Rh sensitization and has reduced the incidence of erythroblastosis fetalis caused by Rh sensitization to 1 per 1,000 live births in the United States.
ABO Incompatibility
ABO incompatibility occurs when a mother with type O blood carries a fetus with type A or B blood. This condition is confined to mothers with type O blood because these women carry anti-A and anti-B IgG antibodies that cross the placenta. Mothers with type A or type B blood produce mostly IgM antibodies against their respective antigens, and these IgM antibodies fail to cross the placenta. Because A and B antigens are common in nature, group O mothers are previously sensitized to these antigens and hemolysis may occur in the first pregnancy. Although ABO incompatibility occurs in 15% of all pregnancies, ABO hemolytic disease occurs in less than 5% of ABO-incompatible mother-infant pairs. Hemolysis tends to be less severe than with Rh incompatibility. The classic presentation is anemia, reticulocytosis, and hyperbilirubinemia occurring in the first 24 to 72 hours of life.
RBC Enzyme Abnormalities
Glucose is the primary metabolic substrate for the red cell, and because the mature red cell lacks organelles including mitochondria, glucose can only be metabolized
via anaerobic pathways. Defects in glucose metabolism or pathways that protect against red cell oxidation can result in hemolysis and hyperbilirubinemia.
via anaerobic pathways. Defects in glucose metabolism or pathways that protect against red cell oxidation can result in hemolysis and hyperbilirubinemia.
Glucose-6-Phosphate Dehydrogenase Deficiency
G6PD is the most common red cell enzyme deficiency, occurring most commonly in infants of Mediterranean, Middle Eastern, African, and Southeast Asian descent. This disorder occurs in up to 15% of African American newborns. The mode of inheritance is X-linked recessive. Presence of this deficiency confers resistance against Plasmodium falciparum, the infectious agent causing malaria. G6PD generates substrates that protect the red cell against oxidative stress. Erythrocytes lacking these substrates undergo hemolysis with oxidative stress. Important triggers in the neonate include sepsis or exposure to other stressors (naphthalene, sulfa drugs, acetaminophen, breast milk of a mother who has eaten fava beans, etc.). However, the pathogenesis of hyperbilirubinemia appears to be more complicated than simple hemolysis. Often, the offending agent is not identified, and infants with significant hyperbilirubinemia have no evidence of hemolysis, leading some authorities to postulate a concurrent impairment in bilirubin conjugation or clearance. Classically, hyperbilirubinemia appears between 24 and 96 hours postpartum, and it can be severe, even in the absence of significant anemia. Up to 50% of patients also have Gilbert syndrome (see later), and this combination increases the risk of hyperbilirubinemia and kernicterus. Many states include G6PD in the routine newborn screen. In the 2004 American Academy of Pediatrics (AAP) guidelines for hyperbilirubinemia, the committee emphasized the possibility for the underdiagnosis of G6PD deficiency in the newborn period, and encouraged practitioners to consider this diagnosis in infants with the appropriate ethnic backgrounds who are poor responders to phototherapy.
Pyruvate Kinase Deficiency
Deficiency in pyruvate kinase causes ineffective adenosine triphosphate generation from glucose, resulting in shortened red cell survival. This disorder is inherited in an autosomal recessive pattern and is most common in whites of northern European descent. Clinical expression ranges from severe neonatal jaundice to compensated hemolytic anemia.
RBC Membrane Defects
Defects that affect the red cell membrane and cytoskeletal structure alter the shape and deformability of the cell and result in hemolysis and hyperbilirubinemia. Conditions include hereditary spherocytosis, elliptocytosis, and pyknocytosis.
Hereditary Spherocytosis
With an incidence of 1 in 5,000, hereditary spherocytosis is the most common congenital RBC membrane disorder. This disease is inherited in an autosomal
dominant manner, with 25% of cases arising from new mutations. Family history can be significant for splenectomy, gallstones, and anemia. Increased RBC Load Any condition that results in increased RBC load can result in hyperbilirubinemia secondary to breakdown of extravascular or sequestered erythrocytes. Common conditions include cephalohematoma, extensive bruising after traumatic delivery, and polycythemia.
dominant manner, with 25% of cases arising from new mutations. Family history can be significant for splenectomy, gallstones, and anemia. Increased RBC Load Any condition that results in increased RBC load can result in hyperbilirubinemia secondary to breakdown of extravascular or sequestered erythrocytes. Common conditions include cephalohematoma, extensive bruising after traumatic delivery, and polycythemia.
Infants of Diabetic Mothers
Macrosomic infants of insulin-dependent diabetic mothers represent a unique group of patients at risk for hyperbilirubinemia. These infants have high levels of erythropoietin and increased erythropoiesis; thus ineffective red cell production and polycythemia are probably responsible for the resulting hyperbilirubinemia.
Decreased Bilirubin Conjugation or Excretion
Hormone Deficiency
The pathogenesis of hyperbilirubinemia in infants with hypothyroidism and hypopituitarism is poorly understood. Hypothyroidism results in unconjugated hyperbilirubinemia, whereas hypopituitarism can result in either unconjugated or conjugated hyperbilirubinemia.
Bilirubin Metabolism Disorder
Bilirubin uridine diphosphate-glucuronosyltransferase (UDPGT) is responsible for converting unconjugated bilirubin into the conjugated form. Defects in this enzyme can result in unconjugated hyperbilirubinemia.
Crigler-Najjar Syndromes, Types I and II
Type I is a rare disease that results from an autosomal recessive inheritance of a deficiency in UDPGT activity. These patients develop severe hyperbilirubinemia in the first 2 to 3 days of life and often require exchange transfusion. Patients with type II disease (also known as Arias syndrome) retain some UDPGT activity, and infants typically have less severe hyperbilirubinemia. Furthermore, infants with type II disease are also partially responsive to phenobarbital, which induces enzyme activity.
Gilbert Syndrome
Gilbert syndrome affects 5% to 10% of the general population, and both autosomal dominant and recessive patterns of inheritance are described. Infants may have mildly elevated levels of unconjugated bilirubin when compared to controls because of decreased UGT1 activity. Presentation with pathologic hyperbilirubinemia in the newborn period is rare, unless a concurrent condition such as G6PD deficiency or hereditary spherocytosis exists.
Lucey-Driscoll Syndrome
Lucey-Driscoll syndrome can be associated with significant hyperbilirubinemia and is thought to be related to a hormone or antibody from the maternal serum that inhibits the action of UDPGT. This is a self-limited process and should be considered in unexplained familial severe hyperbilirubinemia.
Sepsis
Sepsis results in hyperbilirubinemia secondary to hemolysis and impaired conjugation. Patients with sepsis may have both elevated unconjugated and conjugated bilirubin levels. The presence of a urinary tract infection has been specifically associated with jaundice presenting after 8 days of age or an elevated conjugated fraction of bilirubin.
Increased Enterohepatic Circulation of Bilirubin
Breast-Feeding Failure Jaundice and Breast-Milk Jaundice
 HINT: Breast-feeding failure jaundice is early onset of jaundice, occurring in the first 2 to 4 days of life, whereas breast-milk jaundice is responsible for later-onset or persistence of jaundice from the end of the first week through the first few weeks of life.
HINT: Breast-feeding failure jaundice is early onset of jaundice, occurring in the first 2 to 4 days of life, whereas breast-milk jaundice is responsible for later-onset or persistence of jaundice from the end of the first week through the first few weeks of life.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree