Intraventricular Hemorrhage of the Preterm Infant
Laura R. Ment
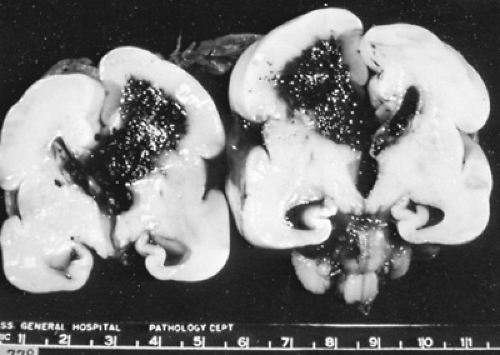
Intraventricular hemorrhage (IVH), or hemorrhage into the germinal matrix tissues with possible rupture into the ventricular system and parenchyma of the developing brain (Fig. 32.1), remains a major problem of preterm neonates. It is believed to be the result of alterations in cerebral blood flow (CBF) to the immature germinal matrix capillary bed. Because the germinal matrix begins to involute after 34 weeks of gestation, germinal matrix hemorrhages (GMHs) and IVH are lesions of preterm infants, and the incidence of GMH/IVH in infants of less than 1,500-g birth weight is now reported to range from 15% to 30%. Seizures, hydrocephalus, periventricular leukomalacia (PVL), and neonatal death are more frequent in infants with GMH/IVH than in those without hemorrhage, when matched for birth weight or gestational age. In addition, although the long-term neurodevelopmental outcome for those infants with lower grades of hemorrhage remains unclear, most observers agree that infants with parenchymal involvement of hemorrhage are at high risk for neurodevelopmental handicap.
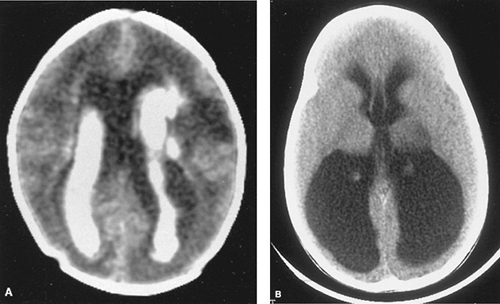
Cranial ultrasonography (Fig. 32.2) is the method of choice for diagnosis of GMH/IVH in newborn special care units. A standard grading system, which originally was applied to the computed tomographic scan, has been adapted to cranial ultrasonography examinations. Grade I (or GMH) describes blood in the germinal matrix only, grade II is blood filling the lateral ventricles without distention, grade III is blood filling and distending the ventricular system, and grade IV describes hemorrhages with parenchymal involvement (Table 32.1). The most common site for parenchymal involvement of hemorrhage is the frontal region; many hemorrhages occur bilaterally. Less commonly, the caudate nuclei and occipital periventricular white matter regions are also involved.
PATHOPHYSIOLOGY
IVH is believed to be secondary to alterations in cerebral blood flow to the germinal matrix microvasculature, and the pathogenesis of GMH is most certainly multifactorial.
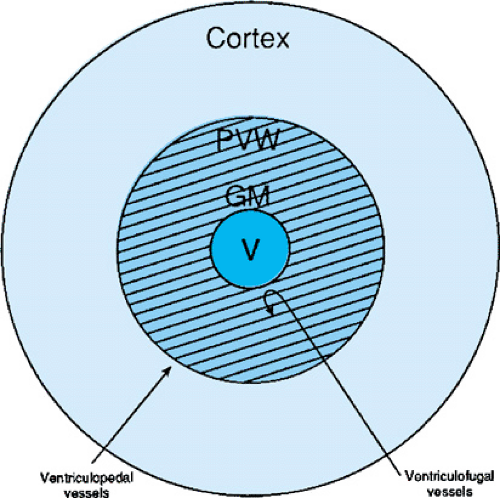
The germinal matrix, the region of origin of GMH/IVH, is the site of proliferation of neuronal and glial precursors in the developing nervous system that, during the late second and early third trimesters, give rise predominantly to glia and microneurons. By 25 weeks’ gestation, almost all the cortical neurons have been generated, axonal and dendritic branching is robust, and synaptic contacts are beginning to form. At this time the capillary bed of the germinal matrix is composed of large, irregular vessels with little evidence for basement membrane proteins or glial supporting structures. In addition, although the blood–brain barrier begins to form during the early third trimester of gestation, the triad of endothelial tight junction formation, basement membrane deposition, and glial investiture does not characterize the microvessels of the germinal matrix zone.
Cerebral blood flow plays a critical role in the pathogenesis of IVH. Although studies in neonatal animals have suggested that the efficiency of cerebral blood flow autoregulation increases with gestational age, cerebral blood flow to the germinal matrix microvessels is largely pressure passive. In this condition, cerebral blood flow varies directly with changes in systemic blood pressure. Thus, if the preterm infant is hypotensive secondary to either in utero hypoxia, sepsis, or other postnatal events, CBF may fall and damage to the germinal matrix microvessels may ensue. In addition, respiratory distress syndrome, vigorous resuscitation, hypoxemia, acidosis, pneumothoraces, and seizures have all been shown to increase CBF in preterm neonates, and the association of these risk factors and hemorrhage has been well described.
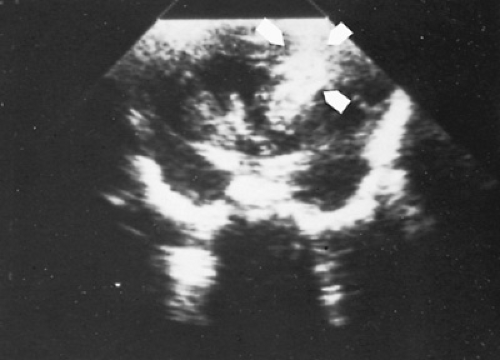 FIGURE 32.2. Coronal ultrasound scan demonstrating a large right parenchymal hemorrhage in a 28-week gestational age, preterm infant with bloody spinal fluid. |
TABLE 32.1. GRADING SYSTEM FOR NEONATAL INTRAVENTRICULAR HEMORRHAGE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
The relationship among surfactant, respiratory distress syndrome, and the development of GMH/IVH also must be mentioned. Although surfactant has been demonstrated to cause transient increases in CBF, most clinical studies have not noted an increase in GMH/IVH associated with its use. In addition, because surfactant may diminish the acute hypoxemia and hypercapnia associated with respiratory distress syndrome, it actually may contribute to the lower incidence of GMH/IVH that some centers are reporting.
Several authors have suggested that the venous circulation may also predispose the germinal matrix to hemorrhage. At the head of the caudate nucleus, the most common site of IVH, the anteriorly coursing terminal, choroidal, and thalamostriate veins join to form the internal cerebral vein. At that juncture, the internal cerebral vein turns abruptly posteriorly to join the vein of Galen. Elevations in venous pressure resulting from neonatal complications such as respiratory distress syndrome, pneumothoraces, or high-frequency ventilation may increase the potential for venous distension in this fragile venous system and thus permit venous hemorrhagic infarction of the germinal matrix tissues.
Finally, the two other risk factors, the absence of antenatal steroid exposure and neonatal transport, are well-recognized associations with IVH. Steroid exposure accelerates surfactant production and may decrease IVH by decreasing the incidence of respiratory distress syndrome; in addition, steroids are angiostatic and may induce maturation of the germinal matrix microvessels. The risks of neonatal transport have been well described and include hypoxemia, hypercarbia, hypotension, and the need for vigorous resuscitation.
Equally concerning and perhaps more important for the neurodevelopmental outcome of these infants is the observation of markedly diminished CBF after low-grade IVH. Xenon-133 inhalation CBF studies and positron emission tomography have demonstrated prolonged ischemia for more than the first postnatal week in preterm neonates with IVH.
A model for the development of neonatal GMH/IVH is found in Fig. 32.3 and takes into account both the hypotensive–ischemic insults and those clinical events, such as
pneumothoraces, seizures, and rapid volume resuscitation, to which many tiny and frequently critically ill preterm neonates are exposed.
pneumothoraces, seizures, and rapid volume resuscitation, to which many tiny and frequently critically ill preterm neonates are exposed.
The neuropathologic consequences of IVH include germinal matrix destruction, periventricular hemorrhagic infarction, and posthemorrhagic hydrocephalus (PHH). Germinal matrix destruction with secondary cystic formation is a common and expected feature of GMH.
In addition, 10% to 20% of patients with GMH/IVH have periventricular hemorrhagic infarction, also frequently called hemorrhagic intracerebral involvement, or grade IV IVH. Some investigators feel that the parenchymal involvement of insult readily visible by cranial ultrasonography (see Fig. 32.2) represents a direct extension of hemorrhage from either the ventricular system or the germinal matrix; others believe that these lesions represent venous infarction of the periventricular white matter. Both proposed mechanisms depend on increased intracranial and (particularly) intraventricular pressure as a primary event. Distinguishing the two lesions in vivo is extremely difficult; likely, parenchymal lesions occur secondary to both mechanisms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree