Internal (Direct) Electronic Monitoring
The fetus can be monitored electronically by direct or indirect methods. Direct fetal heart measurement is accomplished by attaching a bipolar spiral electrode directly to the fetus (Fig. 24-1). The wire electrode penetrates the fetal scalp, and the second pole is a metal wing on the electrode. Vaginal body fluids create a saline electrical bridge that completes the circuit and permits measurement of the voltage differences between the two poles. The two wires of the bipolar electrode are attached to a reference electrode on the maternal thigh to eliminate electrical interference. The electrical fetal cardiac signal—P wave, QRS complex, and T wave—is amplified and fed into a cardiotachometer for heart rate calculation. The peak R-wave voltage is the portion of the fetal electrocardiogram most reliably detected.
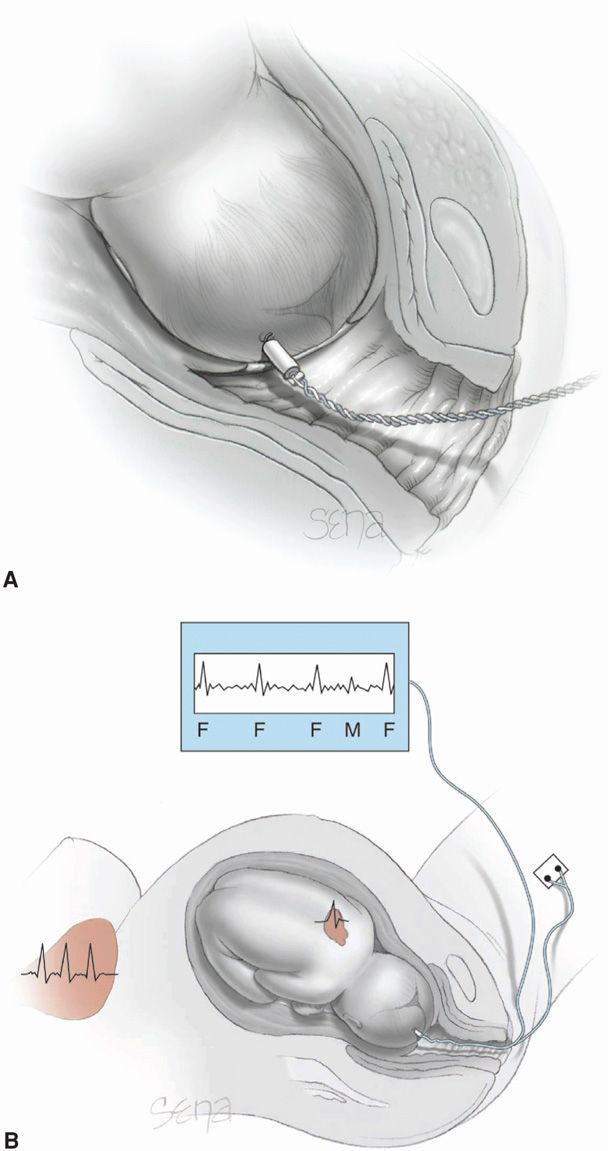
FIGURE 24-1 Internal electronic fetal monitoring. A. Scalp electrode penetrates the fetal scalp by means of a coiled electrode. B. Schematic representation of a bipolar electrode attached to the fetal scalp for detection of fetal QRS complexes (F). Also shown is the maternal heart and corresponding electrical complex (M) that is detected.
An example of the method of fetal heart rate processing employed when a scalp electrode is used is shown in Figure 24-2. Time (t) in milliseconds between fetal R waves is fed into a cardiotachometer, where a new fetal heart rate is set with the arrival of each new R wave. As also shown in Figure 24-2, a premature atrial contraction is computed as a heart rate acceleration because the interval (t2) is shorter than the preceding one (t1). The phenomenon of continuous R-to-R wave fetal heart rate computation is known as beat-to-beat variability. The physiological event being counted, however, is not a mechanical event corresponding to a heartbeat but rather an electrical event.
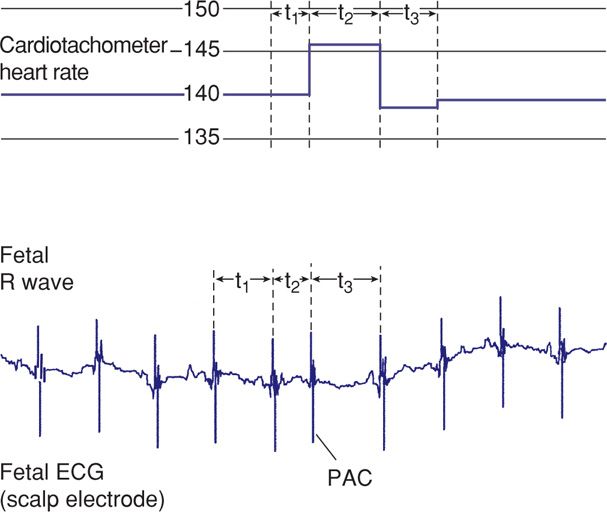
FIGURE 24-2 Schematic representation of fetal electrocardiographic signals used to compute continuing beat-to-beat heart rate with scalp electrodes. Time intervals (t1, t2, t3) in milliseconds between successive fetal R waves are used by a cardiotachometer to compute instantaneous fetal heart rate. ECG = electrocardiogram; PAC = premature atrial contraction.
Electrical cardiac complexes detected by the electrode include those generated by the mother. Although the maternal electrocardiogram (ECG) signal is approximately five times stronger than the fetal ECG, its amplitude is diminished when it is recorded through the fetal scalp electrode. In a live fetus, this low maternal ECG signal is detected but masked by the fetal ECG. If the fetus is dead, the weaker maternal signal will be amplified and displayed as the “fetal” heart rate (Freeman, 2003). Shown in Figure 24-3 are simultaneous recordings of maternal chest wall ECG signals and fetal scalp electrode ECG signals. This fetus is experiencing premature atrial contractions, which cause the cardiotachometer to rapidly and erratically seek new heart rates, resulting in the “spiking” shown in the standard fetal monitor tracing. Importantly, when the fetus is dead, the maternal R waves are still detected by the scalp electrode as the next best signal and are counted by the cardiotachometer (Fig. 24-4).
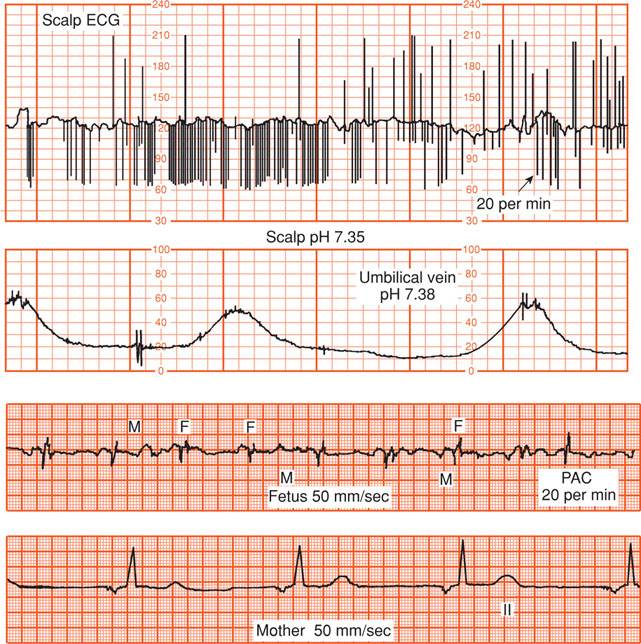
FIGURE 24-3 The top tracing shows standard fetal monitor tracing of heart rate using a fetal scalp electrode. Spiking of the fetal rate in the monitor tracing is due to premature atrial contractions. The second panel displays accompanying contractions. The bottom two tracings represent cardiac electrical complexes detected from fetal scalp and maternal chest wall electrodes. ECG = electrocardiogram; F = fetus; M = mother; PAC = fetal premature atrial contraction.
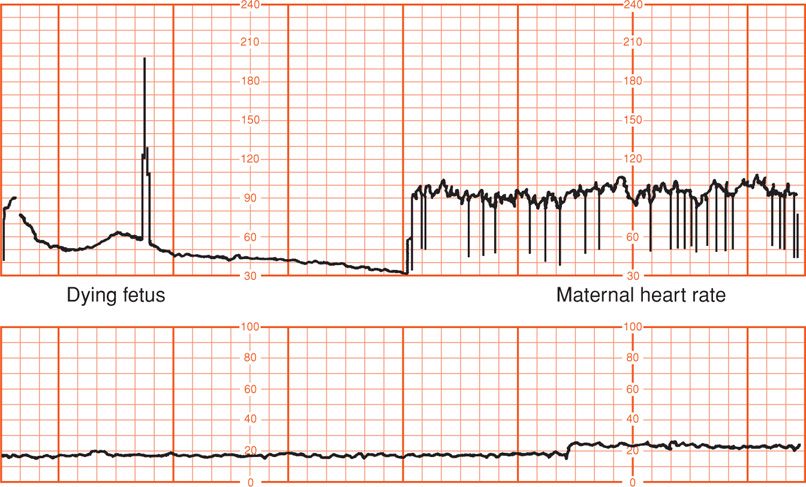
FIGURE 24-4 Placental abruption. In the upper panel, the fetal scalp electrode first detected the heart rate of the dying fetus. After fetal death, the maternal electrocardiogram complex is detected and recorded. The second panel displays an absence of uterine contractions.
 External (Indirect) Electronic Monitoring
External (Indirect) Electronic Monitoring
Membrane rupture may be avoided by use of external detectors to monitor fetal heart action. External monitoring, however, does not provide the precision of fetal heart rate measurement afforded by internal monitoring (Nunes, 2014).
The fetal heart rate is detected through the maternal abdominal wall using the ultrasound Doppler principle (Fig. 24-5). Ultrasound waves undergo a shift in frequency as they are reflected from moving fetal heart valves and from pulsatile blood ejected during systole (Chap. 10, p. 209). The unit consists of a transducer that emits ultrasound and a sensor to detect a shift in frequency of the reflected sound. The transducer is placed on the maternal abdomen at a site where fetal heart action is best detected. A coupling gel must be applied because air conducts ultrasound waves poorly. The device is held in position by a belt. Care should be taken that maternal arterial pulsations are not confused with fetal cardiac motion (Neilson, 2008).
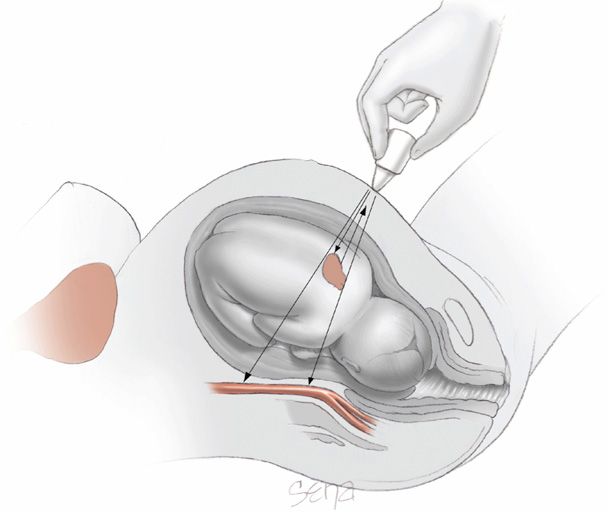
FIGURE 24-5 Ultrasound Doppler principle used externally to measure fetal heart motions. Pulsations of the maternal aorta also may be detected and erroneously counted. (Adapted from Klavan, 1977.)
Ultrasound Doppler signals are edited electronically before fetal heart rate data are printed onto monitor paper. Reflected ultrasound signals from moving fetal heart valves are analyzed through a microprocessor that compares incoming signals with the most recent previous signal. This process, called autocorrelation, is based on the premise that the fetal heart rate has regularity whereas “noise” is random and without regularity. Several fetal heart motions must be deemed electronically acceptable by the microprocessor before the fetal heart rate is printed. Such electronic editing has greatly improved the tracing quality of the externally recorded fetal heart rate.
 Fetal Heart Rate Patterns
Fetal Heart Rate Patterns
It is now generally accepted that interpretation of fetal heart rate patterns can be problematic because of the lack of agreement on definitions and nomenclature (American College of Obstetricians and Gynecologists, 2013b). In one example, Blackwell and colleagues (2011) asked three maternal-fetal medicine specialists to independently interpret 154 fetal heart rate tracings. Interobserver agreement was poor for the most ominous tracings and “moderate” for less severe patterns. The authors cautioned that their results represented idealized circumstances that should not be considered reflective of routine clinical practice.
The National Institute of Child Health and Human Development (NICHD) Research Planning Workshop (1997) brought together investigators with expertise in the field to propose standardized, unambiguous definitions for interpretation of fetal heart rate patterns during labor. This workshop was repeated in 2008. The definitions proposed and shown in Table 24-1 as a result of this second workshop are used in this chapter. First, it is important to recognize that interpretation of electronic fetal heart rate data is based on the visual pattern of the heart rate as portrayed on chart recorder graph paper. Thus, the choice of vertical and horizontal scaling greatly affects the appearance of the fetal heart rate. Scaling factors recommended by the workshop are 30 beats per minute (beats/min or bpm) per vertical cm (range, 30 to 240 bpm) and 3 cm/min chart recorder paper speed. Fetal heart rate variation is falsely displayed at the slower 1 cm/min paper speed compared with that of the smoother baseline recorded at 3 cm/min (Fig. 24-6). Thus, pattern recognition can be considerably distorted depending on the scaling factors used.
TABLE 24-1. Electronic Fetal Monitoring Definitions


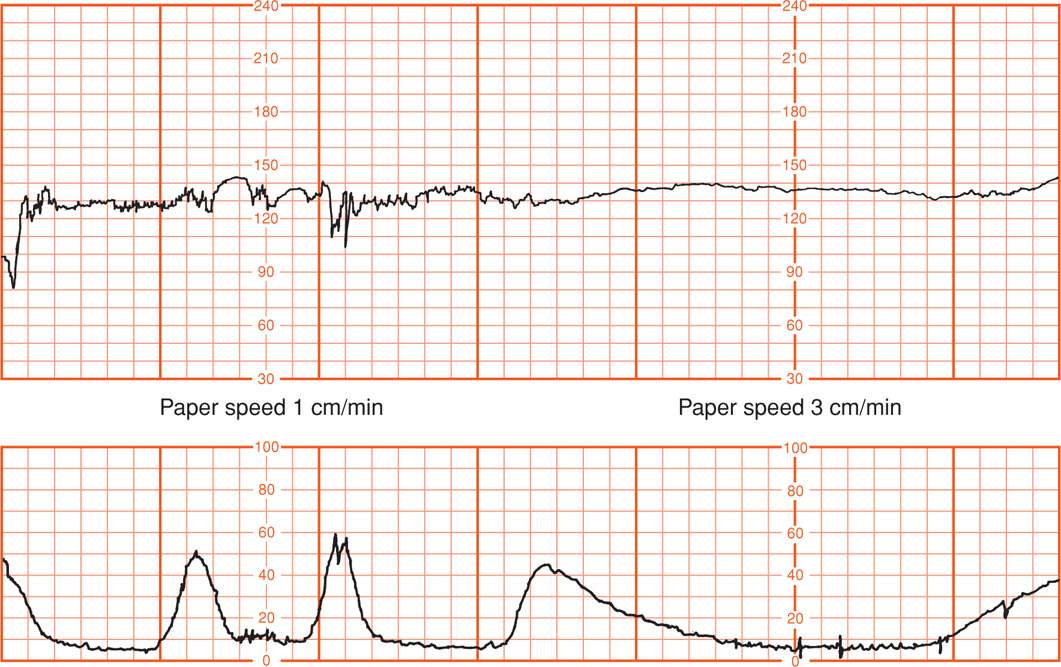
FIGURE 24-6 Fetal heart rate obtained by scalp electrode (upper panel) and recorded at 1 cm/min compared with that at 3 cm/min chart recorder paper speed. Concurrent uterine contractions are shown (lower panel).
 Baseline Fetal Heart Activity
Baseline Fetal Heart Activity
This refers to the modal characteristics that prevail apart from periodic accelerations or decelerations associated with uterine contractions. Descriptive characteristics of baseline fetal heart activity include rate, beat-to-beat variability, fetal arrhythmia, and distinct patterns such as sinusoidal or saltatory fetal heart rates.
Rate
With increasing fetal maturation, the heart rate decreases. This continues postnatally such that the average rate is 90 bpm by age 8 (Behrman, 1992). Pillai and James (1990) longitudinally studied fetal heart rate characteristics in 43 normal pregnancies. The baseline fetal heart rate decreased an average of 24 bpm between 16 weeks and term, or approximately 1 beat/min per week. This normal gradual slowing of the fetal heart rate is thought to correspond to maturation of parasympathetic (vagal) heart control (Renou, 1969).
The baseline fetal heart rate is the approximate mean rate rounded to increments of 5 bpm during a 10-minute tracing segment. In any 10-minute window, the minimum interpretable baseline duration must be at least 2 minutes. If the baseline fetal heart rate is less than 110 bpm, it is termed bradycardia. If the baseline rate is greater than 160 bpm, it is termed tachycardia. The average fetal heart rate is considered the result of tonic balance between accelerator and decelerator influences on pacemaker cells. In this concept, the sympathetic system is the accelerator influence, and the parasympathetic system is the decelerator factor mediated via vagal slowing of heart rate (Dawes, 1985). Heart rate also is under the control of arterial chemoreceptors such that both hypoxia and hypercapnia can modulate rate. More severe and prolonged hypoxia, with a rising blood lactate level and severe metabolic acidemia, induces a prolonged fall in heart rate (Thakor, 2009).
Bradycardia. In the third trimester, the normal mean baseline fetal heart rate has generally been accepted to range between 120 and 160 bpm. The lower normal limit is disputed internationally, with some investigators recommending 110 bpm (Manassiev, 1996). Pragmatically, a rate between 100 and 119 bpm, in the absence of other changes, usually is not considered to represent fetal compromise. Such low but potentially normal baseline heart rates also have been attributed to head compression from occiput posterior or transverse positions, particularly during second-stage labor (Young, 1976). Such mild bradycardias were observed in 2 percent of monitored pregnancies and averaged approximately 50 minutes in duration. Freeman and associates (2003) have concluded that bradycardia within the range of 80 to 120 bpm with good variability is reassuring. Interpretation of rates less than 80 bpm is problematic, and such rates generally are considered nonreassuring.
Some causes of fetal bradycardia include congenital heart block and serious fetal compromise (Jaeggi, 2008; Larma, 2007). Figure 24-7 shows bradycardia in a fetus dying from placental abruption. Maternal hypothermia under general anesthesia for repair of a cerebral aneurysm or during maternal cardiopulmonary bypass for open-heart surgery also can cause fetal bradycardia. Sustained fetal bradycardia in the setting of severe pyelonephritis and maternal hypothermia also has been reported (Hankins, 1997). These infants apparently are not harmed by several hours of such bradycardia.
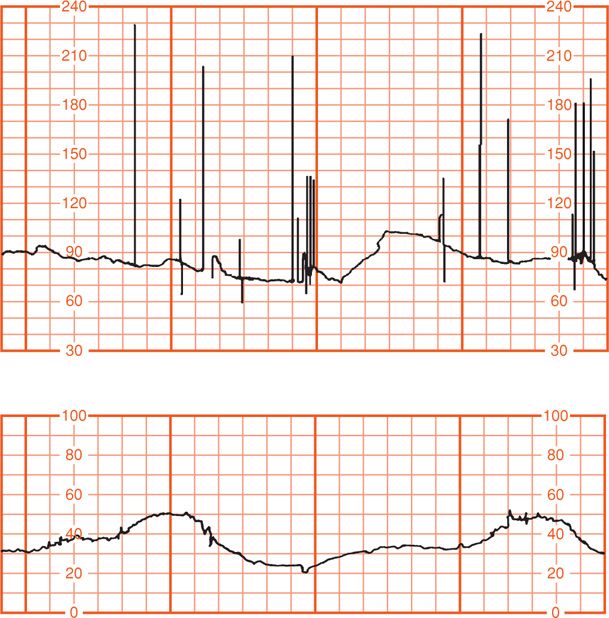
FIGURE 24-7 Fetal bradycardia measured with a scalp electrode (upper panel) in a pregnancy complicated by placental abruption and subsequent fetal death. Concurrent uterine contractions are shown in the lower panel.
Tachycardia. Fetal tachycardia is defined as a baseline heart rate greater than 160 bpm. The most common explanation for fetal tachycardia is maternal fever from chorioamnionitis, although fever from any source can increase baseline fetal heart rate. Such infections also have been observed to induce fetal tachycardia before overt maternal fever is diagnosed (Gilstrap, 1987). Fetal tachycardia caused by maternal infection typically is not associated with fetal compromise unless there are associated periodic heart rate changes or fetal sepsis.
Other causes of fetal tachycardia include fetal compromise, cardiac arrhythmias, and maternal administration of parasympathetic (atropine) or sympathomimetic (terbutaline) drugs. The key feature to distinguish fetal compromise in association with tachycardia seems to be concomitant heart rate decelerations. Prompt relief of the compromising event, such as correction of maternal hypotension caused by epidural analgesia, can result in fetal recovery.
Wandering Baseline. This baseline rate is unsteady and “wanders” between 120 and 160 bpm (Freeman, 2003). This rare finding is suggestive of a neurologically abnormal fetus and may occur as a preterminal event.
Beat-to-Beat Variability
Baseline variability is an important index of cardiovascular function and appears to be regulated largely by the autonomic nervous system (Kozuma, 1997). That is, a sympathetic and parasympathetic “push and pull” mediated via the sinoatrial node produces moment-to-moment or beat-to-beat oscillation of the baseline heart rate. Such heart rate change is defined as baseline variability. Variability can be further divided into short term and long term, although these terms have fallen out of use. Short-term variability reflects the instantaneous change in fetal heart rate from one beat—or R wave—to the next. This variability is a measure of the time interval between cardiac systoles (Fig. 24-8). Short-term variability can most reliably be determined to be normally present only when electrocardiac cycles are measured directly with a scalp electrode. Long-term variability is used to describe the oscillatory changes during 1 minute and result in the waviness of the baseline (Fig. 24-9). The normal frequency of such waves is three to five cycles per minute (Freeman, 2003).
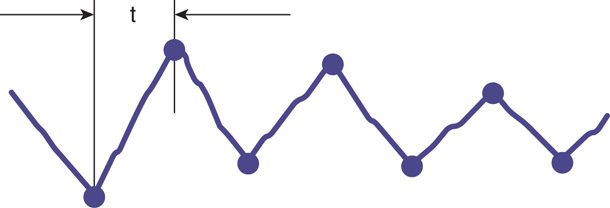
FIGURE 24-8 Schematic representation of short-term beat-to-beat variability measured by a fetal scalp electrode. t = time interval between successive fetal R waves. (Adapted from Klavan, 1977.)
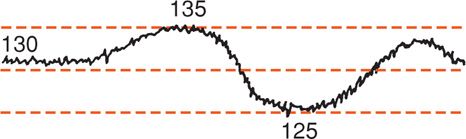
FIGURE 24-9 Schematic representation of long-term beat-to-beat variability of the fetal heart rate ranging between 125 and 135 bpm. (Adapted from Klavan, 1977.)
It should be recognized that precise quantitative analysis of both short- and long-term variability presents a number of frustrating problems due to technical and scaling factors. For example, Parer and coworkers (1985) evaluated 22 mathematical formulas designed to quantify heart rate variability and found most to be unsatisfactory. Consequently, most clinical interpretation is based on visual analysis with subjective judgment of the smoothness or flatness of the baseline. According to Freeman and colleagues (2003), there is no current evidence that the distinction between short- and long-term variability has any clinical relevance. Similarly, the NICHD Workshop (1997) did not recommend differentiating short- and long-term variability because in actual practice they are visually determined as a unit. The workshop panel defined baseline variability as those baseline fluctuations of two cycles per minute or greater. They recommended the criteria shown in Figure 24-10 for quantification of variability. Normal beat-to-beat variability was accepted to be 6 to 25 bpm.
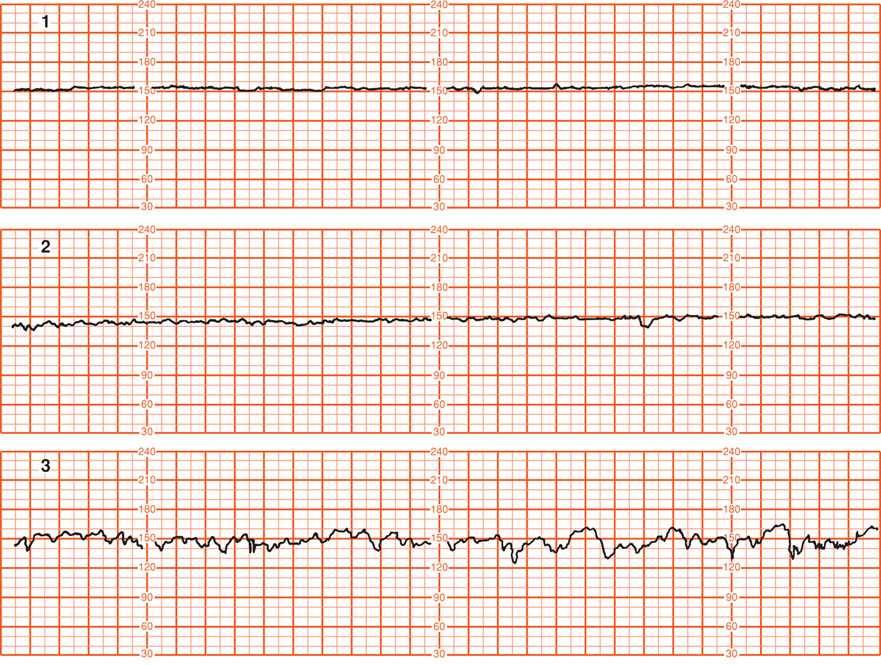
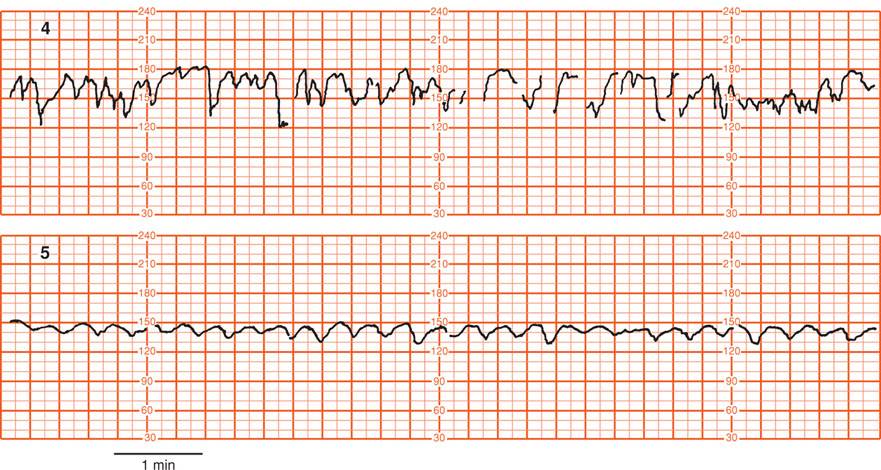
FIGURE 24-10 Grades of baseline fetal heart rate variability shown in the following five panels. 1. Undetectable, absent variability. 2. Minimal variability, ≤ 5 bpm. 3. Moderate (normal) variability, 6 to 25 bpm. 4. Marked variability, > 25 bpm. 5. Sinusoidal pattern. This differs from variability in that it has a smooth, sinelike pattern of regular fluctuation and is excluded in the definition of fetal heart rate variability. (Adapted from National Institute of Child Health and Human Development Research Planning Workshop, 1997.)
Increased Variability. Several physiological and pathological processes can affect or interfere with beat-to-beat variability. Dawes and associates (1981) described increased variability during fetal breathing. In healthy infants, short-term variability is attributable to respiratory sinus arrhythmia (Divon, 1986). Fetal body movements also affect variability (Van Geijn, 1980). Pillai and James (1990) reported increased baseline variability with advancing gestation. Up to 30 weeks, baseline characteristics were similar during both fetal rest and activity. After 30 weeks, fetal inactivity was associated with diminished baseline variability and conversely, variability was increased during fetal activity. Fetal gender does not affect heart rate variability (Ogueh, 1998).
The baseline fetal heart rate becomes more physiologically fixed (less variable) as the rate increases. Conversely, there is more instability or variability of the baseline at lower heart rates. This phenomenon presumably reflects less cardiovascular physiological wandering as beat-to-beat intervals shorten due to increasing heart rate.
Decreased Variability. Diminished beat-to-beat variability can be an ominous sign indicating a seriously compromised fetus. Paul and coworkers (1975) reported that loss of variability in combination with decelerations was associated with fetal acidemia. They analyzed variability in the 20 minutes preceding delivery in 194 pregnancies. Decreased variability was defined as 5 or fewer bpm excursion of the baseline (see Fig. 24-10), whereas acceptable variability exceeded this range. Fetal scalp pH was measured 1119 times in these pregnancies, and mean values were found to be increasingly acidemic when decreased variability was added to progressively intense heart rate decelerations. For example, mean fetal scalp pH of approximately 7.10 was found when severe decelerations were combined with 5 bpm or less variability, compared with a pH of approximately 7.20 when greater variability was associated with similarly severe decelerations. Severe maternal acidemia also can cause decreased fetal beat-to-beat variability, as shown in Figure 24-11 in a mother with diabetic ketoacidosis.
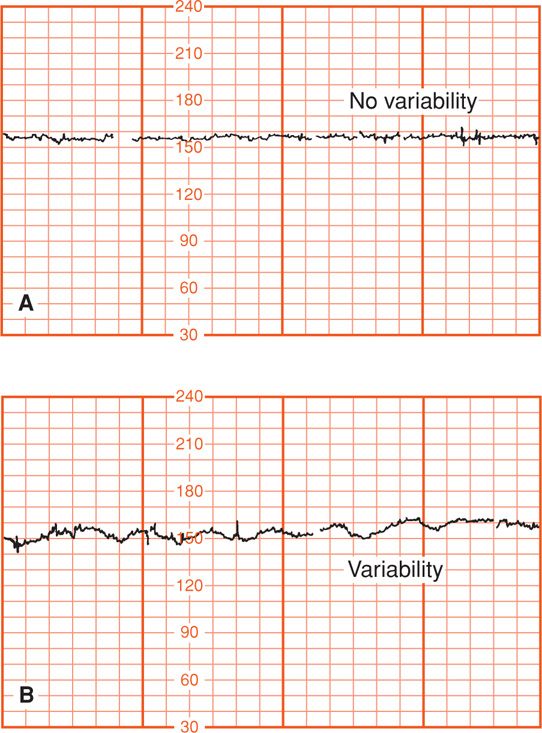
FIGURE 24-11 A. External fetal heart recording showing lack of long-term variability at 31 weeks during maternal diabetic ketoacidosis (pH 7.09). B. Recovery of fetal long-term variability after correction of maternal acidemia.
The precise pathological mechanisms by which fetal hypoxemia results in diminished beat-to-beat variability are not totally understood. Interestingly, mild degrees of fetal hypoxemia have been reported actually to increase variability, at least at the outset of the hypoxic episode (Murotsuki, 1997). According to Dawes (1985), it seems probable that the loss of variability is a result of metabolic acidemia that causes depression of the fetal brainstem or the heart itself. Thus, diminished beat-to-beat variability, when it reflects fetal compromise, likely reflects acidemia rather than hypoxia.
A common cause of diminished beat-to-beat variability is administration of analgesic drugs during labor (Chap. 25, p. 506). Various central nervous system depressant drugs can cause transient diminished beat-to-beat variability. Included are narcotics, barbiturates, phenothiazines, tranquilizers, and general anesthetics. Variability regularly diminishes within 5 to 10 minutes following intravenous meperidine administration, and the effects may last up to 60 minutes or longer depending on the dosage given (Petrie, 1993). Butorphanol given intravenously diminishes fetal heart rate reactivity (Schucker, 1996). In a study performed at Parkland Hospital, Hill and colleagues (2003) found that 5 bpm or less variability occurred in 30 percent of women given continuous intravenous meperidine compared with 7 percent in those given continuous labor epidural analgesia.
Magnesium sulfate, widely used in the United States for tocolysis as well as management of hypertensive women, has been arguably associated with diminished beat-to-beat variability. Hallak and associates (1999) randomly assigned 34 normal, nonlaboring women to standard magnesium sulfate infusion versus isotonic saline. Magnesium sulfate was associated with statistically decreased variability only in the third hour of the infusion. The average decrease in variability was deemed clinically insignificant, however, because the mean variability was 2.7 bpm in the third hour of magnesium infusion compared with 2.8 bpm at baseline. Magnesium sulfate also blunted the frequency of accelerations.
It is generally believed that reduced baseline heart rate variability is the single most reliable sign of fetal compromise. Smith and coworkers (1988) performed a computerized analysis of beat-to-beat variability in growth-restricted fetuses before labor. They observed that diminished variability (4.2 bpm or less) that was maintained for 1 hour was diagnostic of developing acidemia and imminent fetal death. By contrast, Samueloff and associates (1994) evaluated variability as a predictor of fetal outcome during labor in 2200 consecutive deliveries. They concluded that variability by itself could not be used as the only indicator of fetal well-being. Conversely, they also concluded that good variability should not be interpreted as necessarily reassuring. Blackwell and associates (2011) found that even experts often disagreed as to whether variability was absent or minimal (< 5 beats per minute).
In summary, beat-to-beat variability is affected by various pathological and physiological mechanisms. Variability has considerably different meaning depending on the clinical setting. The development of decreased variability in the absence of decelerations is unlikely to be due to fetal hypoxia (Davidson, 1992). A persistently flat fetal heart rate baseline—absent variability—within the normal baseline rate range and without decelerations may reflect a previous insult to the fetus that has resulted in neurological damage (Freeman, 2003).
Cardiac Arrhythmia
When fetal cardiac arrhythmias are first suspected using electronic monitoring, findings can include baseline bradycardia, tachycardia, or most commonly in our experience, abrupt baseline spiking (Fig. 24-12). Intermittent baseline bradycardia is frequently due to congenital heart block. As discussed in Chapter 59 (p. 1172), conduction defects, most commonly complete atrioventricular (AV) block, usually are found in association with maternal connective-tissue diseases. An arrhythmia can only be documented, practically speaking, when scalp electrodes are used. Some fetal monitors can be adapted to output the scalp electrode signals into an electrocardiographic recorder. Because only a single lead is obtained, analysis and interpretation of rhythm and rate disturbances are severely limited.
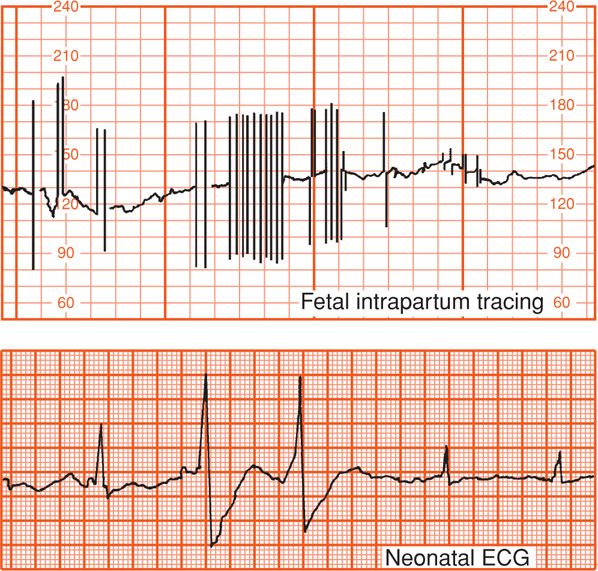
FIGURE 24-12 Internal fetal monitoring at term demonstrated occasional abrupt beat-to-beat fetal heart rate spiking due to erratic extrasystoles shown in the corresponding fetal electrocardiogram. The normal infant was delivered spontaneously and had normal cardiac rhythm in the nursery.
Southall and associates (1980) studied antepartum fetal cardiac rate and rhythm disturbances in 934 normal pregnancies between 30 and 40 weeks. Arrhythmias, episodes of bradycardia < 100 bpm, or tachycardia > 180 bpm were encountered in 3 percent. Most supraventricular arrhythmias are of little significance during labor unless there is coexistent heart failure as evidenced by fetal hydrops. Many supra-ventricular arrhythmias disappear in the immediate neonatal period, although some are associated with structural cardiac defects (Api, 2008). Copel and coworkers (2000) used echocardiography to evaluate 614 fetuses referred for auscultated irregular heart rate without hydrops. Only 10 fetuses (2 percent) were found to have significant arrhythmias, and all but one of these infants survived.
Boldt and colleagues (2003) followed 292 consecutive fetuses diagnosed with a cardiac arrhythmia through birth and into childhood. Atrial extrasystoles were the most common arrhythmia (68 percent), followed by atrial tachycardias (12 percent), atrioventricular block (12 percent), sinus bradycardia (5 percent), and ventricular extrasystoles (2.5 percent). Chromosomal anomalies were found in 1.7 percent of the fetuses. Fetal hydrops developed in 11 percent, and 2 percent had intrauterine death. Fetal hydrops was a bad prognostic finding. Overall, 93 percent of the study population was alive at a median follow-up period of 5 years, and 3 percent—seven infants—had neurological handicaps. Of the infants with atrial extrasystoles, 97 percent lived, and none suffered neurological injury. Only 6 percent required postnatal cardiac medications. Lopriore and associates (2009) found low rates of death and long-term neurological impairment in fetuses with supraventricular tachycardia or atrial flutter. In contrast, higher mortality rates were noted in those with atrioventricular block.
Although most fetal arrhythmias are of little consequence during labor when there is no evidence of fetal hydrops, such arrhythmias impair interpretation of intrapartum heart rate tracings. Sonographic evaluation of fetal anatomy and echocardiography may be useful. Some clinicians use fetal scalp sampling as an adjunct. Generally, in the absence of fetal hydrops, neonatal outcome is not measurably improved by pregnancy intervention. At Parkland Hospital, intrapartum fetal cardiac arrhythmias, especially those associate with clear amnionic fluid, are managed conservatively. Freeman and colleagues (2003) have extensively reviewed interpretation of the fetal electrocardiogram during labor.
Sinusoidal Heart Rate
A true sinusoidal pattern such as that shown in panel 5 of Figure 24-10 may be observed with fetal intracranial hemorrhage, with severe fetal asphyxia, and with severe fetal anemia from Rh alloimmunization, fetomaternal hemorrhage, twin-twin transfusion syndrome, or vasa previa with bleeding (Modanlou, 2004). Insignificant sinusoidal patterns have been reported following administration of meperidine, morphine, alphaprodine, and butorphanol (Angel, 1984; Egley, 1991; Epstein, 1982). Shown in Figure 24-13 is a sinusoidal pattern seen with maternal meperidine administration. An important characteristic of this pattern when due to narcotics is the sine frequency of 6 cycles per minute. A sinusoidal pattern also has been described with chorioamnionitis, fetal distress, and umbilical cord occlusion (Murphy, 1991). Young (1980a) and Johnson (1981) with their coworkers concluded that intrapartum sinusoidal fetal heart patterns were not generally associated with fetal compromise.
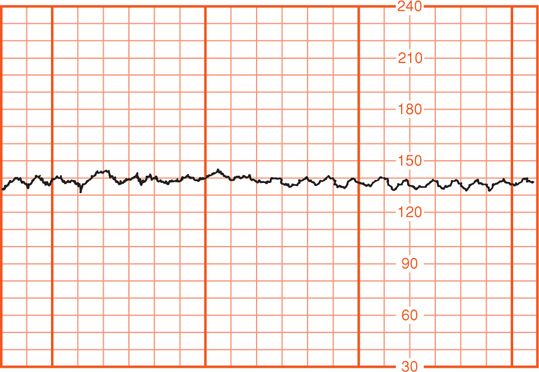
FIGURE 24-13 Sinusoidal fetal heart rate pattern associated with maternal intravenous meperidine administration. Sine waves are occurring at a rate of 6 cycles per minute.
Modanlou and Freeman (1982), based on their extensive review, proposed adoption of a strict definition:
1. Stable baseline heart rate of 120 to 160 bpm with regular oscillations,
2. Amplitude of 5 to 15 bpm (rarely greater),
3. Long-term variability frequency of 2 to 5 cycles per minute,
4. Fixed or flat short-term variability,
5. Oscillation of the sinusoidal waveform above or below a baseline, and
6. Absent accelerations.
Although these criteria were selected to define a sinusoidal pattern that is most likely ominous, they observed that the pattern associated with alphaprodine is indistinguishable. Other investigators have proposed a classification of sinusoidal heart rate patterns into mild—amplitude 5 to 15 bpm, intermediate—16 to 24 bpm, and major—25 or more bpm to quantify fetal risk (Murphy, 1991; Neesham, 1993).
Some investigators have defined intrapartum sine wavelike baseline variation with periods of acceleration as pseudosinusoidal. Murphy and colleagues (1991) reported that pseudosinusoidal patterns were seen in 15 percent of monitored labors. Mild pseudosinusoidal patterns were associated with use of meperidine and epidural analgesia. Intermediate pseudosinusoidal patterns were linked to fetal sucking or transient episodes of fetal hypoxia caused by umbilical cord compression. Egley and associates (1991) reported that 4 percent of fetuses demonstrated sinusoidal patterns transiently during normal labor. These authors observed patterns for up to 90 minutes in some cases and also in association with oxytocin or alphaprodine usage, or both.
The pathophysiology of sinusoidal patterns is unclear, in part due to various definitions. There seems to be general agreement that antepartum sine wave baseline undulation portends severe fetal anemia. Still, few D-alloimmunized fetuses develop this pattern (Nicolaides, 1989). The sinusoidal pattern has been reported to develop or disappear after fetal transfusion (Del Valle, 1992; Lowe, 1984). Ikeda and associates (1999) have proposed, based on studies in fetal lambs, that the sinusoidal fetal heart rate pattern is related to waves of arterial blood pressure, reflecting oscillations in the baroreceptor-chemoreceptor feedback mechanism for control of the circulation.
 Periodic Fetal Heart Rate Changes
Periodic Fetal Heart Rate Changes
The periodic fetal heart rate refers to deviations from baseline that are temporally related to uterine contractions. Acceleration refers to an increase in fetal heart rate above baseline and deceleration to a decrease below the baseline rate. The nomenclature most commonly used in the United States is based on the timing of the deceleration in relation to contractions—thus, early, late, or variable in onset related to the corresponding uterine contraction. The waveform of these decelerations is also significant for pattern recognition. In early and late decelerations, the slope of fetal heart rate change is gradual, resulting in a curvilinear and uniform or symmetrical waveform. With variable decelerations, the slope of fetal heart rate change is abrupt and erratic, giving the waveform a jagged appearance. The 1997 workshop proposed that decelerations be defined as recurrent if they occur with 50 percent or more of contractions in any 20-minute period.
Another system now used less often to describe decelerations is based on the pathophysiological events considered most likely to cause the pattern. In this system, early decelerations are termed head compression, late decelerations are termed uteroplacental insufficiency, and variable decelerations become cord compression patterns.
Accelerations
These are visually apparent abrupt increases—defined as onset of acceleration to a peak in less than 30 seconds—in the fetal heart rate baseline (American College of Obstetricians and Gynecologists, 2013b). At 32 weeks’ gestation and beyond, the acceleration has a peak of 15 bpm with a duration of 15 seconds or more but less than 2 minutes (see Table 24-1). Before 32 weeks, a peak of 10 bpm for 15 seconds to 2 minutes is considered normal. Prolonged acceleration was defined as 2 minutes or more but less than 10 minutes.
According to Freeman and coworkers (2003), accelerations most often occur antepartum, in early labor, and in association with variable decelerations. Proposed mechanisms for intrapartum accelerations include fetal movement, stimulation by uterine contractions, umbilical cord occlusion, and fetal stimulation during pelvic examination. Fetal scalp blood sampling and acoustic stimulation also incite fetal heart rate acceleration (Clark, 1982). Finally, accelerations can occur during labor without any apparent stimulus. Indeed, they are common in labor and are nearly always associated with fetal movement. These accelerations are virtually always reassuring and almost always confirm that the fetus is not acidemic at that time.
Accelerations seem to have the same physiological explanations as beat-to-beat variability in that they represent intact neurohormonal cardiovascular control mechanisms linked to fetal behavioral states. Krebs and colleagues (1982) analyzed electronic heart rate tracings in nearly 2000 fetuses and found sporadic accelerations during labor in 99.8 percent. Fetal heart accelerations during the first or last 30 minutes during labor, or both, was a favorable sign for fetal well-being. The absence of such accelerations during labor, however, is not necessarily an unfavorable sign unless coincidental with other nonreassuring changes. There is an approximately 50-percent chance of acidemia in the fetus who fails to respond to stimulation in the presence of an otherwise nonreassuring pattern (Clark, 1984; Smith, 1986).
Early Deceleration
This consists of a gradual decrease and return to baseline associated with a contraction (Fig. 24-14). Such early deceleration was first described by Hon (1958), who observed that there was a heart rate drop with contractions and that this was related to cervical dilatation. He considered these findings to be physiological.
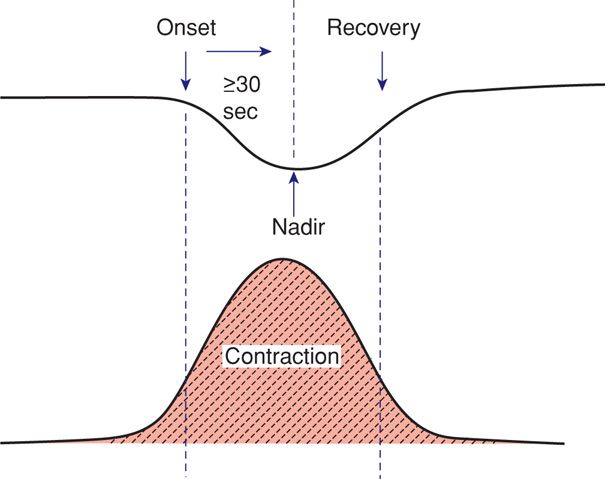
FIGURE 24-14 Features of early fetal heart rate deceleration. Characteristics include a gradual decline in the heart rate with both onset and recovery coincident with the onset and recovery of the contraction. The nadir of the deceleration is 30 seconds or more after the deceleration onset.
Freeman and associates (2003) defined early decelerations as those generally seen in active labor between 4 and 7 cm dilatation. In their definition, the degree of deceleration is generally proportional to the contraction strength and rarely falls below 100 to 110 bpm or 20 to 30 bpm below baseline. Such decelerations are common during active labor and not associated with tachycardia, loss of variability, or other fetal heart rate changes. Importantly, early decelerations are not associated with fetal hypoxia, acidemia, or low Apgar scores.
Head compression probably causes vagal nerve activation as a result of dural stimulation, and this mediates the heart rate deceleration (Paul, 1964). Ball and Parer (1992) concluded that fetal head compression is a likely cause not only of the deceleration shown in Figure 24-14 but also of those shown in Figure 24-15, which typically occur during second-stage labor. Indeed, they observed that head compression is the likely cause of many variable decelerations classically attributed to cord compression.
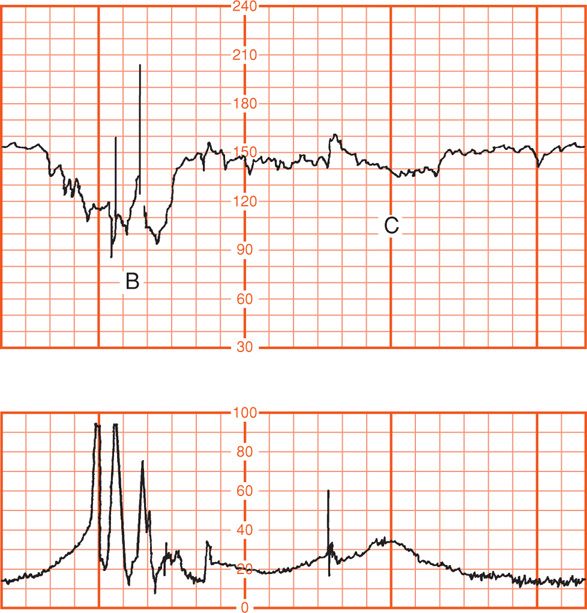
FIGURE 24-15 Two different fetal heart rate patterns during second-stage labor that are likely both due to head compression (upper panel). Maternal pushing efforts (lower panel) correspond to the spikes with uterine contractions. Fetal heart rate deceleration (C) is consistent with the pattern of head compression shown in Figure 24-14. Deceleration (B), however, is “variable” in appearance because of its jagged configuration and may alternatively represent cord occlusion.
Late Deceleration
The fetal heart rate response to uterine contractions can be an index of either uterine perfusion or placental function. A late deceleration is a smooth, gradual, symmetrical decrease in fetal heart rate beginning at or after the contraction peak and returning to baseline only after the contraction has ended. A gradual decrease is defined as 30 seconds or more from the onset of the deceleration to the nadir. In most cases, the onset, nadir, and recovery of the deceleration occur after the beginning, peak, and ending of the contraction, respectively (Fig. 24-16). The magnitude of late decelerations is seldom more than 30 to 40 bpm below baseline and typically not more than 10 to 20 bpm. Late decelerations usually are not accompanied by accelerations.
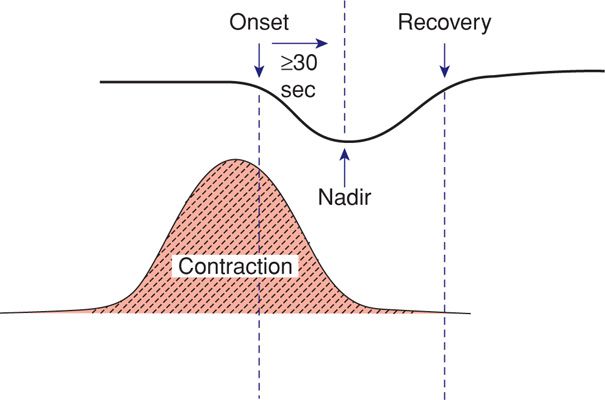
FIGURE 24-16 Features of late fetal heart rate deceleration. Characteristics include gradual decline in the heart rate with the contraction nadir, and recovery occurring after the end of the contraction. The nadir of the deceleration occurs 30 seconds or more after the onset of the deceleration.
Myers and associates (1973) studied monkeys in which they compromised uteroplacental perfusion by lowering maternal aortic blood pressure. The interval or lag from the contraction onset until the late deceleration onset was directly related to basal fetal oxygenation. They demonstrated that the length of the lag phase was predictive of the fetal Po2 but not fetal pH. The lower the fetal Po2 before contractions, the shorter the lag phase to onset of late decelerations. This lag period reflected the time necessary for the fetal Po2 to fall below a critical level necessary to stimulate arterial chemoreceptors, which mediated decelerations.
Murata and coworkers (1982) also showed that a late deceleration was the first fetal heart rate consequence of uteroplacental-induced hypoxia. During the course of progressive hypoxia that led to death over 2 to 13 days, monkey fetuses invariably exhibited late decelerations before development of acidemia. Variability of the baseline heart rate disappeared as acidemia developed.
Numerous clinical circumstances can result in late decelerations. Generally, any process that causes maternal hypotension, excessive uterine activity, or placental dysfunction can induce late decelerations. The two most common causes are hypotension from epidural analgesia and uterine hyperactivity caused by oxytocin stimulation. Maternal diseases such as hypertension, diabetes, and collagen-vascular disorders can cause chronic placental dysfunction. Placental abruption can cause acute late decelerations (Fig. 24-17).
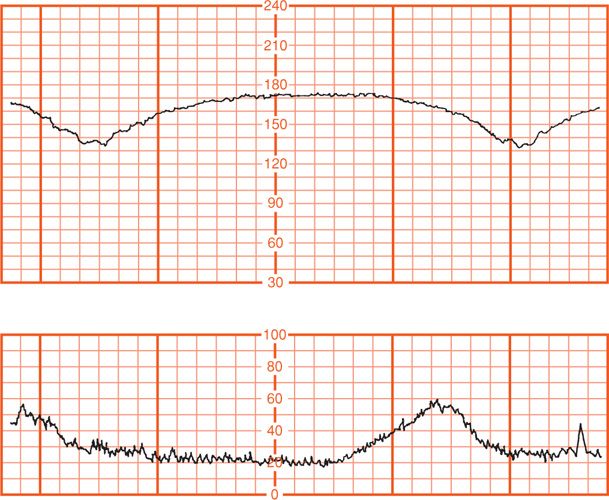
FIGURE 24-17 Late decelerations due to uteroplacental insufficiency resulting from placental abruption. Immediate cesarean delivery was performed. Umbilical artery pH was 7.05 and the Po2 was 11 mm Hg.
Variable Deceleration
The most common deceleration patterns encountered during labor are variable decelerations attributed to umbilical cord occlusion. Melchior and Bernard (1985) identified variable decelerations in 40 percent of more than 7000 monitor tracings when labor had progressed to 5 cm dilatation and in 83 percent by the end of the first stage of labor. Variable deceleration is defined as an abrupt decrease in the fetal heart rate beginning with the onset of the contraction and reaching a nadir in less than 30 seconds. The decrease must last between ≥ 15 seconds and 2 minutes and must be ≥ 15 bpm in amplitude. The onset of deceleration typically varies with successive contractions (Fig. 24-18).
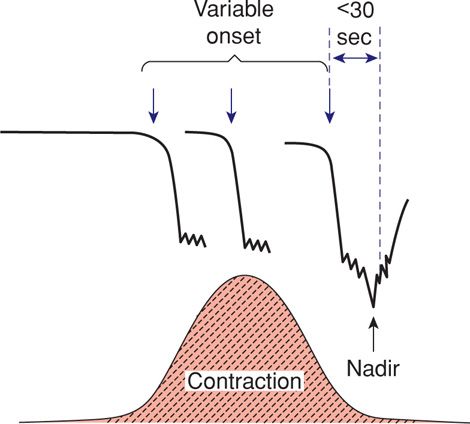
FIGURE 24-18 Features of variable fetal heart rate decelerations. Characteristics include an abrupt decline in the heart rate, and onset that commonly varies with successive contractions. The decelerations measure ≥ 15 bpm for ≥ 15 seconds and have an onset-to-nadir phase of < 30 seconds. Total duration is < 2 minutes.
Very early in the development of electronic monitoring, Hon (1959) tested the effects of umbilical cord compression on fetal heart rate (Fig. 24-19). Similar complete occlusion of the umbilical cord in experimental animals produces abrupt, jagged-appearing deceleration of the fetal heart rate (Fig. 24-20). Concomitantly, fetal aortic pressure increases. Itskovitz and colleagues (1983) observed that variable decelerations in fetal lambs occurred only after umbilical blood flow was reduced by at least 50 percent.
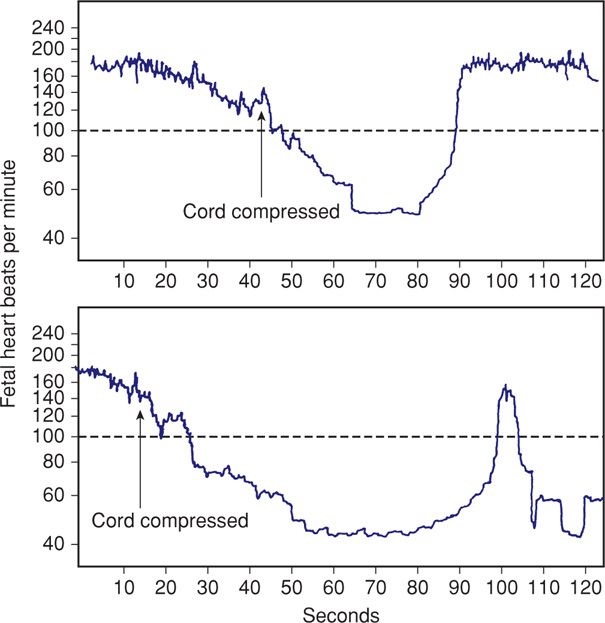
FIGURE 24-19 A. The effects of 25-second cord compression compared with those of 40 seconds in panel (B). (Redrawn from Hon, 1959, with permission.)
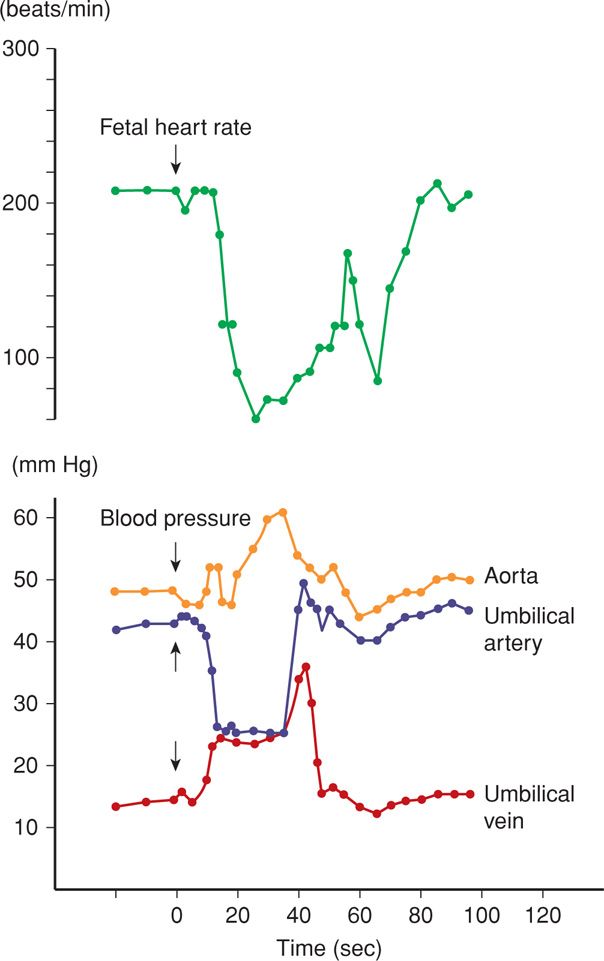
FIGURE 24-20 Total umbilical cord occlusion (arrow) in the sheep fetus is accompanied by an increase in fetal aortic blood pressure. Blood pressure changes in the umbilical vessels are also shown. (Redrawn from Künzel, 1985, with permission.)
Two types of variable decelerations are shown in Figure 24-21. The deceleration denoted by “A” is very much like that seen with complete umbilical cord occlusion in experimental animals (see Fig. 24-20). Deceleration “B,” however, has a different configuration because of the “shoulders” of acceleration before and after the deceleration component. Lee and coworkers (1975) proposed that this form of variable deceleration was caused by differing degrees of partial cord occlusion. In this physiological scheme, occlusion of only the vein reduces fetal blood return, thereby triggering a baroreceptor-mediated acceleration. With increasing intrauterine pressure and subsequent complete cord occlusion, fetal systemic hypertension develops due to obstruction of umbilical artery flow. This stimulates a baroreceptor-mediated deceleration. Presumably, the aftercoming shoulder of acceleration represents the same events occurring in reverse (Fig. 24-22).
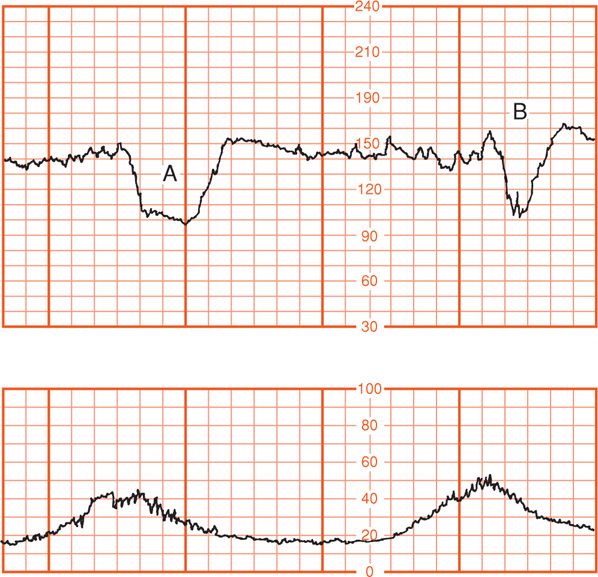
FIGURE 24-21 Varying (variable) fetal heart rate decelerations. Deceleration (B) exhibits “shoulders” of acceleration compared with deceleration (A). (Adapted from Künzel, 1985, with permission.)
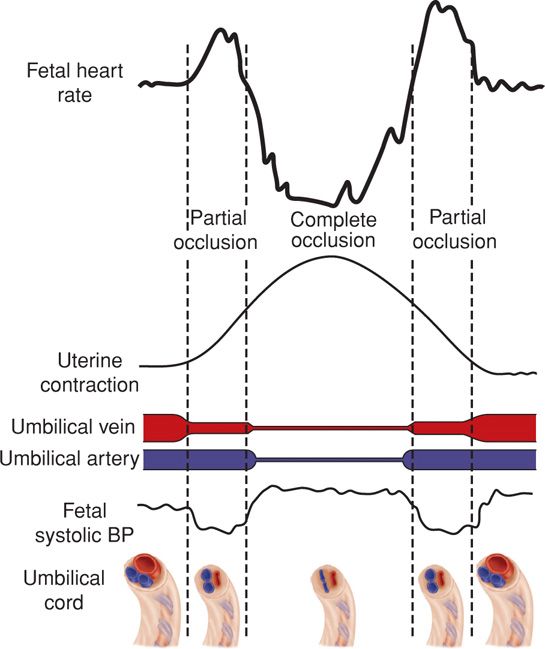
FIGURE 24-22 Schematic representation of the fetal heart rate effects with partial and complete umbilical cord occlusion. Uterine pressures generated early in a contraction cause cord compression predominantly of the thin-walled umbilical vein. The resulting decrease in fetal cardiac output leads to an initial compensatory rise in fetal heart rate. As cord compression intensifies, umbilical arteries are then also compressed. The resulting rise in fetal systolic blood pressure leads to a vagal-mediated fetal heart rate deceleration. As the contraction abates and compression is relieved first on the umbilical arteries, elevated fetal systolic blood pressures drop and the deceleration resolves. A final increase in fetal heart rate is seen as a result of persistent umbilical vein occlusion. With completion of the uterine contraction and cord compression, the fetal heart rate returns to baseline. BP = blood pressure. (Adapted from Lee, 1975.)
Ball and Parer (1992) concluded that variable decelerations are mediated vagally and that the vagal response may be due to chemoreceptor or baroreceptor activity or both. Partial or complete cord occlusion produces an increase in afterload (baroreceptor) and a decrease in fetal arterial oxygen content (chemoreceptor). These both result in vagal activity leading to deceleration. In fetal monkeys, the baroreceptor reflexes appear to operate during the first 15 to 20 seconds of umbilical cord occlusion followed by decline in Po2 at approximately 30 seconds, which then serves as a chemoreceptor stimulus (Mueller-Heubach, 1982).
Thus, variable decelerations represent fetal heart rate reflexes that reflect either blood pressure changes due to interruption of umbilical flow or changes in oxygenation. It is likely that most fetuses have experienced brief but recurrent periods of hypoxia due to umbilical cord compression during gestation. The frequency and inevitability of cord occlusion undoubtedly have provided the fetus with these physiological mechanisms as a means of coping. The great dilemma for the obstetrician in managing variable fetal heart rate decelerations is determining when variable decelerations are pathological. According to the American College of Obstetricians and Gynecologists (2013a), recurrent variable decelerations with minimal to moderate variability are indeterminate, whereas those with absent variability are abnormal.
Other fetal heart rate patterns have been associated with umbilical cord compression. Saltatory baseline heart rate (Fig. 24-23) was first described by Hammacher and colleagues (1968) and linked to umbilical cord complications during labor. The pattern consists of rapidly recurring couplets of acceleration and deceleration causing relatively large oscillations of the baseline fetal heart rate. We also observed a relationship between cord occlusion and the saltatory pattern (Leveno, 1984). In the absence of other fetal heart rate findings, these do not signal fetal compromise. Lambda is a pattern involving an acceleration followed by a variable deceleration with no acceleration at the end of the deceleration. This pattern typically is seen in early labor and is not ominous (Freeman, 2003). This lambda pattern may result from mild cord compression or stretch. Overshoot is a variable deceleration followed by acceleration. The clinical significance of this pattern is controversial (Westgate, 2001).
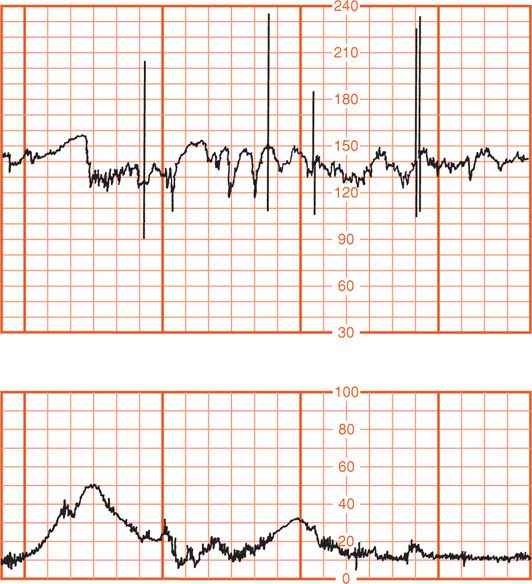
FIGURE 24-23 Saltatory baseline fetal heart rate showing rapidly recurring couplets of acceleration combined with deceleration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


