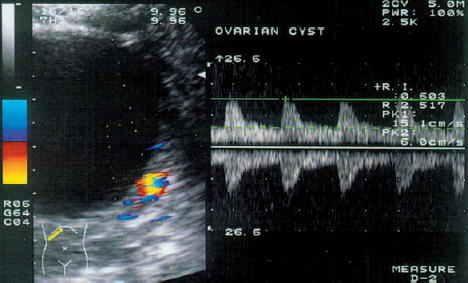
9 Interventional Ultrasound in Reproductive Medicine Laparoscopy was the method of choice for oocyte retrieval in the earliest case reports on successful in-vitro fertilization21,30,31,45,70. Its greatest disadvantage, however, was that follicles maturing deep in the ovary or in an ovary concealed by matted adhesions could not be directly visualized55. Moreover, laparoscopy usually required general anesthesia and was associated with increased perioperative morbidity and mortality. Another problem was that the pneumoperitoneum created with carbon dioxide led to very slight, transient pH changes that could have a harmful effect on the oocytes. In the early 1980 s, the first generation of real-time scanners offered sufficiently high resolution for the percutaneous ultrasound-guided aspiration of oocytes. Lenz et al.40 were the first authors to describe ultrasound-guided follicular aspiration. Analgesic and sedative premedication allowed for a painless procedure with high patient acceptance39. General anesthesia was not used because it might induce hyperprolactinemia, which could adversely affect the microenvironment of the oocyte60. Equipment. Early studies described the use of linear transducers75, whereas most operators today prefer sector scanners. High-resolution transducers have a separate biopsy attachment with corresponding software. Most authors use a 16-gauge needle with an inside diameter of 1.1 mm43, 75. The Teflon coating on the needle counteracts the adhesive tendency of the oocytes, while the sharp needle tip can easily pierce the ovarian tissue with minimal pressure or pain. To flush the follicle, the proximal end of the needle is connected by Teflon tubing to a syringe filled with warmed, heparinized culture medium. Flushing the follicle after it has been aspirated helps to maximize the oocyte recovery rate per punctured follicle60. The irrigation lumen may be parallel or concentric to the aspiration lumen. The first attempts employed manual aspiration with a 5 ml syringe. But since the uncontrolled pressure of manual aspiration could cause rupture of the zone pellucida, operators developed a foot-controlled mechanical aspiration pump with a maximum pressure of 80–100 mmHg14. Other equipment includes a comfortable operating table, sterile drapes and protective sleeves, sterile ultrasound gel, and a water bath or heating unit. Patient preparation. Every patient requires thorough counseling and meticulous preparation. A large number of different stimulation protocols are used to induce the growth of multiple follicles. A combination of endocrinological and sonographic data are used to determine the timing of hCG (human chorionic gonadotrophin) administration. An ultrasound examination is advised before the start of the procedure. The goal of this examination is to determine the number and size of the follicles and compare them with the data on the day of hCG administration. The patient should drink 2 liters of liquid one hour before the procedure to distend the bladder. In some cases it may still be necessary to insert a transurethral catheter and instill Hartmann solution into the bladder, and the operator should understand the associated risks of iatrogenic infection and bladder irritation. A distended bladder permits clear visualization of the ovarian follicles and moves the bowel out of the area between the abdominal wall and ovaries. Combined analgesic and sedative premedication is given to most patients, and the needle insertion site is infiltrated with local anesthetic. Other options are peridural or spinal anesthesia. The lower abdomen is prepared with an antiseptic solution, and the operative field is packed off with sterile drapes. The endovaginal transducer is smeared with sterile ultrasound gel and covered with a sterile protective sleeve. The control panel of the ultrasound unit is covered with sterile, transparent plastic film. Technique. The puncture needle is inserted directly into the bladder using freehand technique or a needle guide. After the needle has pierced the posterior bladder wall and ovarian capsule, the needle tip is directed into the center of the closest follicle. The follicular fluid is aspirated, and the collapsed follicle is flushed with an equal volume of culture medium. The needle tip is kept within the ovary, and all the follicles are systematically aspirated using the same technique. The operator should understand that inadequate bladder filling or pelvic adhesions can alter the position of the ovaries and that overweight and heavy scar tissue result in poor visualization of the pelvic organs. After the procedure is completed, the patient may empty her bladder and can usually be discharged home two hours later. Parsons50 and Dellenbach17 developed the transurethral technique of oocyte retrieval. The procedure was done on an outpatient basis to reduce costs. The patient was placed in the lithotomy position with the operator on her right, the ultrasound cart on her left, and an assistant seated in the middle between the patient’s legs9. The vulva was aseptically prepared with chlorhexidine solution. The needle tip was introduced through the side opening of the Foley catheter and passed through the urethra into the bladder. After the bladder was filled with Hartmann solution, the catheter cuff was inflated and the needle tip was advanced out of the catheter. The operator then passed the needle tip through the posterior bladder wall under sonographic guidance and directed it into the nearest follicle. All the follicles were successively aspirated and flushed. Finally the needle was withdrawn, the bladder was emptied, and the catheter was removed. The patient drank several glasses of liquid and emptied her bladder before leaving the hospital. The overall complication rate was very low. There were no complaints of cystitis or adnexitis following the procedure. Booker et al.8 compared the transurethral route for ultrasound-guided follicular aspiration with the transvaginal route in a randomized prospective study. Considerably more follicles could be visualized by the transvaginal route, but there were no differences in the number of oocytes harvested, in the duration of the procedure, or in the fertility, embryo-transfer or pregnancy rates. This led the authors to conclude that the transurethral and transvaginal routes were of equal value for ultrasound-guided follicular aspiration. Gleicher et al.25 were the first authors to report on transvaginal follicular aspiration guided by abdominal ultrasound. A speculum is placed in the vagina, and the needle is passed through the fornix to the ovary. Subsequent experience has shown that the transvaginal approach guided with an endovaginal transducer is superior to all other ultrasound-guided techniques22. Advantages. The proximity of the transducer to the pelvic organs enables the use of high-frequency transducers that provide significantly better resolution and greater clinical efficiency. Owing to the natural compliance of the fornix, the tip of the transducer can be moved closer to the ovaries by applying gentle pressure. Since a full bladder is not required, the position of the pelvic organs is unchanged and the ovaries are located within the focal zone of the transducer. Overweight or adhesions do not prevent visualization of the follicles and thus are not a contraindication to this method. Standard stimulation protocols are monitored with the aid of transvaginal sonography32. Additional information can be gained by hormone assays and by the color Doppler assessment of blood flow in the uterus and ovary34,37,38. Preparations. The entire treatment is done as an outpatient procedure. The patient is placed in the lithotomy position. Sedatives (such as flunitrazepam, droperidol, or pentazocine) can be given, but approximately 50% of IVF groups do not use anesthesia or sedation23. Since follicular aspiration takes about 10 minutes on average, most patients tolerate the procedure with no difficulties. Nevertheless, the operator should be aware of possible hypotensive reactions or queasiness. After ultrasound gel has been applied to the transducer probe, the protective sleeve (a sterile condom, surgical glove, or specially designed sheath) is placed over the gel-smeared probe, avoiding any air bubbles that might cause artifacts. The gel should not be used to lubricate the probe insertion because of its spermicidal and reputed embryotoxic properties58. Physiological saline solution or culture medium should be used instead. Technique. The vagina is irrigated with isotonic saline solution or culture medium, and the transducer probe is inserted into the vagina. A sterile needle guide is used for transvaginal follicular aspiration. An automatic puncture device has been developed to avoid the potential risks of needle insertion. This device consists of a movable metal tube and a guide mechanism into which the aspiration needle is inserted and secured22. The device should be “locked and loaded” before it is inserted into the vagina with the transducer. After the insertion, a detailed sonographic examination is done to locate the uterus and ovaries. The probe is positioned so that the puncture line indicating the needle path is aimed precisely at the center of the nearest follicle. The operator calculates the exact distance along the puncture line to the targeted follicle and “fires” the needle into the follicle. The follicular fluid is then aspirated into a test tube connected to the aspiration pump. The collapse of the follicle can be observed during aspiration of the follicular fluid22. Flushing of the aspirated follicle may be done to increase the number of retrieved oocytes. This is done by infusing culture medium with heparin added through the puncture needle or through a separate line. Without removing the needle, the operator aspirates all of the follicles that are situated along the puncture line. Complications. Feichtinger et al.24 described a low complication rate with ultrasound-guided follicular aspiration. In 2.4% of cases the iliac vein was mistaken for a follicle and erroneously punctured. Ultrasound revealed intraperitoneal bleeding into the cul-de-sac, but this resolved spontaneously in all cases. It was shown that filling the bladder can stop the bleeding by exerting pressure on the puncture site. The use of color Doppler may provide a simple way to avoid this complication, as the iliac vessels are easily visualized with this technique. Bleeding from the posterior vaginal wall is easy to diagnose and can be stopped by compression. Pelvic inflammatory disease (PID) is considered a rare complication of transvaginal follicular aspiration (0.14% of all patients)24. In most cases the infection was caused by bacterially contaminated semen and occurred in women with a prior history of PID. GIFT. The GIFT procedure was developed to overcome various obstacles to spermatozoon transport and the failure of the fallopian tube to capture the oocyte during ovulation3. Patients in this program undergo ovarian stimulation under ultrasound surveillance. The oocytes are harvested by laparoscopy, identified in the laboratory, and transferred to a catheter containing 100 000 spermatozoa collected by the swim-up technique3. The transfer catheter is introduced into the fimbriated end of the fallopian tube, where its contents are gently emptied. By bringing together the sperm and oocyte, this technique circumvents many of the factors that can disrupt sperm transport and fusion46. Because fertilization occurs in the natural milieu, the success rate is 26.5% deliveries per oocyte retrieval61. ZIFT. ZIFT is an advanced form of GIFT in which the oocytes are harvested by transvaginal aspiration, fertilized in vitro, and transferred the next day in the pronuclear stage into one of the fallopian tubes using the GIFT technique62. The transvaginal aspiration technique used in the ZIFT method is the same as that described for IVF. The overall success rate appears to be identical to that of GIFT and IVF. Advantages and disadvantages. Disadvantages of the GIFT and ZIFT procedures are that they cannot be used in patients with tubal pathology and they require anesthesia and operating room technique. Also, neither procedure can be used in patients with male factor infertility since fertilization is uncertain. One refinement is to transfer the spermatozoa and oocytes into the fallopian tube by transcervical catheterization of the tube, but this method is associated with the same pregnancy rates with no significant reduction in costs or risks. Intrauterine embryo transfer represents the last critical step in in-vitro fertilization. Inadequate transfer of the embryo into the uterine cavity can be an important factor in the failure of implantation. For this reason, ultrasound-guided embryo transfer might offer significant advantages over the traditional “blind” transfer27. Hurley et al.29 performed intrauterine embryo transfer with a transcervical catheter in 94 patients using transvaginal ultrasound guidance. The culture medium that contained the embryos was injected along with air bubbles so that the embryos could be optimally placed in the uterine cavity under visual guidance. This method was particularly helpful in six cases where the catheter became “stuck” in the endocervical canal, contrary to the operator’s impression that the catheter was accurately placed in the uterine cavity. This method has also proved effective in patients with submucous leiomyomas or uterine anomalies. Moreover, the ultrasound-guided procedure allays anxiety by allowing patients to watch the transfer on the monitor screen. Lenz et al.41 and Parsons et al.49 used transvaginal ultrasound for guidance during transabdominal embryo transfer through the wall of the uterus into the uterine cavity. This method could provide an alternative to transcervical transfer in patients with known cervical stenosis making it difficult to pass a catheter through the cervical canal. Indications and technique. Fifteen percent of female infertility cases are caused by proximal occlusion of the fallopian tubes. The occlusions at that level may result from intraluminal cellular debris, mild adhesions, or muscular spasms64. Transcervical tubal catheterization can be used for both the diagnosis and treatment of infertility patients15,16,33,56,59,68. The criteria used in selecting patients for this procedure are the presence of bilateral tubal occlusions or the stenosis of the remaining tube in patients who have had a unilateral salpingectomy27. The catheter is advanced into the fallopian tube over a guidewire to stretch open the tube. When aided by fluoroscopic guidance, tubal catheterization has a success rate of 98% for proximal occlusions in the area of the uterotubal junction but only 33% for more distal stenoses56. Ultrasound guidance. Transvaginal ultrasound can be used to direct the tubal catheterization, although there has been little experience with this technique to date. Lisse and Sydow44 described tubal catheterization under transvaginal ultrasound guidance. Concomitant laparoscopy confirmed the accurate placement of the catheter in the proximal part of the tube (3–6 cm from the intramural junction). Tubal patency was restored in 85% of the patients with a proximal occlusion. Breckenridge and Schinfeld11 and Thurmond et al.67 recommend transabdominal ultrasound as a simpler method of guiding the catheter insertion than transvaginal ultrasound. They report that the full bladder necessary for abdominal ultrasound straightens the uterus and makes it more easily accessible. Complications. Possible complications of transcervical tubal catheterization are perforation of the fallopian tube, vasovagal reactions during the procedure, and infection. Patients should also be informed of the increased risk of ectopic pregnancy following the procedure. Indications and technique. Transvaginal ultrasound allows the direct visualization and aspiration of persistent follicular cysts27. These cysts can impair folliculogenesis either by hormone secretion or by compressing the tissue and restricting blood flow. For the aspiration of an ovarian or paraovarian cyst, the needle tip is introduced into the center of the cyst. The merits of this procedure are debated in the literature. The fear of seeding cells into the abdominal cavity from a potentially malignant ovarian process has discouraged many operators from using this method. While cytological analysis is always performed on the aspirated cyst fluid, a negative cytological report is occasionally false-negative. The high sensitivity and specificity of transvaginal color Doppler ultrasound in the difference of benign and malignant adnexal masses can apparently aid the decision of which cysts should be aspirated (Fig. 9.1). Recurrence rates. Bret et al.12,13 published two reports on their experience with transvaginal ultrasound in the aspiration of ovarian cysts. They described a recurrence rate after cyst aspiration of 48% in premenopausal patients and 80% in postmenopausal women. Alcohol was injected into the aspirated cyst to prevent recurrence, but this was successful in only four of seven patients12. Vaegemaekers et al.74 performed the transvaginal aspiration of 32 unilocular, hypoechoic ovarian cysts (average diameter 45 mm) in infertile patients. The authors concluded from their study that ovarian cyst aspiration in the early follicular phase might reduce the dropout rate from IVF cycles. Fig. 9.1
Follicular Aspiration in Assisted Reproduction
Transabdominal Follicular Aspiration
Transurethral Follicular Aspiration
Transvaginal Follicular Aspiration
GIFT (Gamete Intrafallopian Transfer) and ZIFT (Zygote Intrafallopian Transfer)
Embryo Transfer
Fallopian Tube Catheterization
Aspiration of Ovarian Cysts
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree