Interventional Cardiology
Phillip Moore
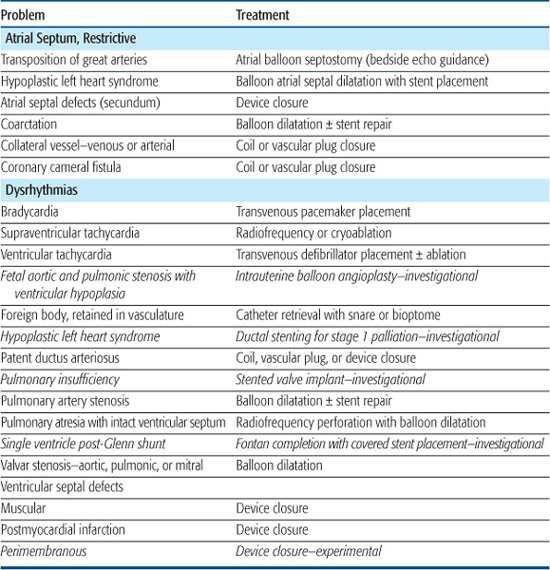
As the diagnostic role of cardiac catheterization has diminished over the years, its role as a mode of primary therapy has exponentially increased. In most large congenital cardiac centers today more than 70% of all children undergoing cardiac catheterization have an interventional therapy as part of the procedure. Interventional cardiac catheterization has become the standard of care for treating an increasing number of congenital heart lesions, while in others it remains investigational (Table 499-1). Common interventional procedures include balloon septostomy in neonates with d-transposition of the great arteries, balloon valvoplasty in valvar pulmonic or aortic stenosis, balloon angioplasty with or without stent repair of branch pulmonary artery stenosis or coarctation of the aorta, device closure of a patent ductus arteriosus, atrial septal defect, muscular ventricular septal defect, and embolization of abnormal venous or arterial vessels. Investigational interventional catheter based treatments include stented valve implant for pulmonary insufficiency, ductal stenting for first stage treatment of infants with hypoplastic left heart syndrome or pulmonary atresia, and fetal intervention for critical aortic or pulmonic stenosis to promote ventricular growth in utero.
Interventional procedures are directed at avoiding, postponing, or complementing surgery with its attendant risks, scars, and lengthy hospitalization and recovery times. Occasionally there is no suitable surgical procedure. Most patients treated with interventional catheterization go home the same day as the procedure and can return to full activity within 5 days. The therapeutic procedures performed in pediatric populations are dilatations, valve implants, or closures. Dilatations are performed using balloons alone or in combination with stents, valve implants use specially designed valved stents, whereas closures are done with embolization coils or specially designed devices or plugs.
Table 499-1. Diseases Treated with Interventional Catheterization Procedures
ENLARGING OR CREATING ATRIAL COMMUNICATIONS
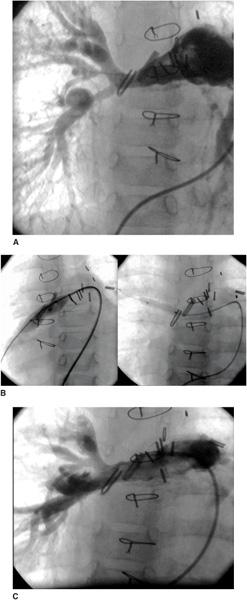
An unrestrictive atrial septal defect may be necessary for patients with certain cardiac abnormalities. Adequate mixing through an atrial septal defect is essential for survival in neonates with d-transposition of the great arteries (dTGA) as well as in patients with a functional single ventricle who have a severe mitral stenosis or atresia, tricuspid atresia, or total anomalous pulmonary venous connection. Balloon atrial septostomy (Rashkind procedure) involves passing a balloon-tipped catheter through the foramen ovale into the left atrium (LA), inflating the balloon, and then pulling it back rapidly across the atrial septum. The resulting tear in the atrial septum usually improves mixing. In most centers, this procedure is performed under echocardiographic guidance and moderate sedation at the bedside (Fig. 499-1). Although balloon catheters successfully tear the thin valve of the foramen ovale of neonates with dTGA, the thicker septum in neonates with left-heart obstruction, or in older infants is not usually amenable to balloon septostomy. Techniques used to open the septum in these patients include static balloon dilatation with high pressure or cutting balloons, and atrial septal stenting (Fig. 499-2).
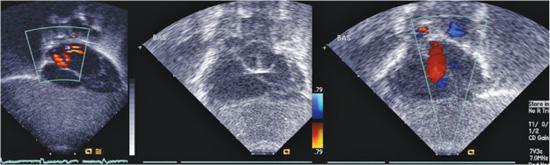
FIGURE 499-1. Balloon atrial septostomy performed under echo guidance at the bedside in an infant with d-transposition of the great arteries. First frame shows intact septum before the procedure. Middle frame shows the Rashkind balloon inflated in the left atrium prior to “jerk” through the septum to the right atrium. Final frame shows a large atrial septic defect now present with left to right color flow.
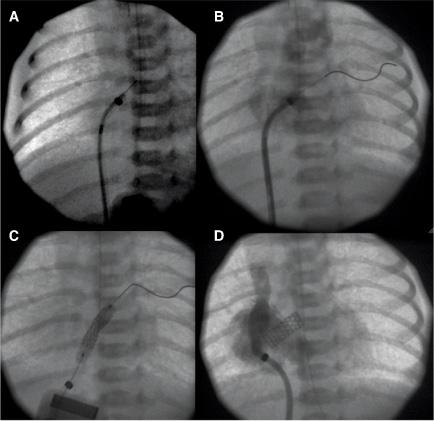
FIGURE 499-2. A: Radiofrequency perforation of the atrial septum in a newborn with hypoplastic left heart syndrome and restrictive atrial septum. B: Wire advanced through perforated septum into left upper pulmonary vein. C: Coronary stent implantation in atrial septum. D: Angiogram after implant showing stable stent position and newly formed atrial communication.
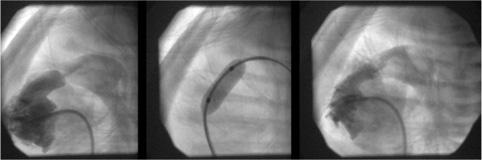
FIGURE 499-3. Lateral angiogram in the right ventricle showing severe pulmonary valvar stenosis in a neomate. Middle panel shows balloon dilatation. Final image shows improved flow through the pulmonary valve into the main pulmonary artery.
VALVOPLASTY
Balloon pulmonary valvoplasty is safe and highly successful in reducing the transvalvar gradient while producing little pulmonary insufficiency. With large balloons that exceed the size of the pulmonary valve annulus by 20% to 40%, pulmonary valve gradients are typically reduced to less than 15 mmHg. The residual obstruction and insufficiency are similar to that seen after surgery. It has become the standard procedure for valvar pulmonic stenosis because the mortality and morbidity are extremely low, it is an outpatient procedure, and there is no sternotomy, cardiopulmonary bypass, blood transfusions, general anesthetic, or scar (Fig. 499-3). Only very thick dysplastic valves, as occur in Noonan syndrome, and supravalvar stenosis may respond poorly to balloon dilatation and require surgery. Even neonates with pulmonary atresia and intact ventricular septum can undergo successful balloon valvoplasty after the atretic valve is perforated by a radio frequency or laser-tipped wire.
For similar reasons balloon aortic valvoplasty is the treatment of choice in patients with valvar aortic stenosis and can be performed with low mortality, good relief of aortic obstruction, and variable but usually mild aortic insufficiency. Patients with calcific aortic stenosis, moderate to severe aortic insufficiency, or annular hypoplasia are not good candidates for valvoplasty. Early and medium-length follow-up studies show results comparable to primary surgery; usually there is less aortic regurgitation but occasionally severe aortic regurgitation necessitates valve replacement. Longer-term studies suggest the durability or repair with balloon dilatation may be less than that with surgery. The risks of valvoplasty include arrhythmias, emboli, neurologic events, and injury to access vessels. In contrast, surgical risks include mortality, risks of anesthesia, cardiopulmonary bypass, blood products, the disadvantages of a long hospitalization, an undesirable scar, and greater cost. Both balloon aortic valvoplasty and surgical valvotomy are palliative. Longer-term follow-up suggests that repeat treatment for either recurrent stenosis or worsening insufficiency occurs in 50% of patients within 8 years of valvoplasty. In some patients who develop restenosis after surgical or balloon procedures, balloon valvoplasty has been used as a means of deferring valve replacement; such delays are particularly important in small children who would otherwise require multiple valve replacements.
Balloon mitral valvoplasty has been performed via a transseptal approach in a few children in the United States, and a larger number in Asia and the Middle East. Preliminary results indicate very good relief of obstruction with minimal resulting mitral insufficiency in children with rheumatic mitral stenosis. Results for congenital mitral stenosis are variable, with a greater propensity for developing mitral insufficiency. Balloon dilatation in this situation is used sparingly, usually reserved for children in whom surgical repair carries an unusually high risk.
BALLOON ANGIOPLASTY
Angioplasty of obstructed vessels in children has been performed since 1982. Relief of obstruction is achieved by tearing the intimal and medial layers. The most commonly involved vessels are arteries, specifically the pulmonary arteries and aorta, though some postoperative venous obstructions are amenable to dilatation as well. Recent advances in balloon catheter technology, allowing for smaller balloon catheters that can be inflated to extremely high pressures (25 atm), have made these procedures safe and more effective. Balloons with tiny surgical blades attached, called cutting balloons, are now available. They allow dilatation of even the most resistant vessel stenosis. For some lesions, such as distal pulmonary artery stenosis, direct surgical relief is extremely difficult, making angioplasty clearly the treatment of choice.
Stenosis of the branch pulmonary arteries may be congenital, acquired, or postsurgical (ie, at shunt insertion sites, bands, or conduits). Examples of congenital pulmonary artery branch stenosis (PABS) include tetralogy of Fallot with pulmonary atresia, Williams syndrome, Alagille syndrome, and congenital rubella. Stenosis caused by scarring from previous Blalock-Taussig or other aortopulmonary shunts is usually amenable to balloon palliation. Native stenoses or hypoplastic pulmonary arteries can be less responsive, although with the current improved balloon technology success rates are greater than 90% (Fig. 499-4). In some patients there may be modest initial improvement in the size of the pulmonary arteries, which then show significant growth thereafter. A combined surgical-transcatheter approach to patients with complex pulmonary artery stenosis has now become commonplace. Surgery to proximal stenoses and any intracardiac defects is used in conjunction with transcatheter therapy of distal pulmonary stenosis and recurrent obstruction improves short- and long-term outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


