Injectable Contraception
Depot-medroxyprogesterone acetate (Depo-Provera) is the most thoroughly studied progestin-only contraceptive. Although its approval for contraception in the United States is relatively recent (1992), it has been available in some countries since the mid-1960s. Much of our knowledge of the safety, efficacy, and acceptability of long-acting hormonal contraception comes from Indonesia, Sri Lanka, Thailand, and Mexico where depot-medroxyprogesterone acetate has been used and studied for decades. The long-delayed approval as a contraceptive in the United States was based on political and economic considerations, not scientific ones.1
Depot-medroxyprogesterone acetate is formulated as microcrystals, suspended in an aqueous solution. The correct dose for contraceptive purposes is 150 mg intramuscularly (gluteal or deltoid) every 3 months. A comparative trial established that the 100-mg dose is significantly less effective.2 The contraceptive level is maintained for at least 14 weeks, providing a safety margin for one of the most effective contraceptives available, about one pregnancy per 100 women after 5 years of consistent use.2,3
A newer formulation allows the self-administration into a thigh or the abdomen of a subcutaneous dose of 104 mg every 3 months.4,5,6 Prefilled syringes contain 0.65 mL of an aqueous suspension of medroxyprogesterone acetate.
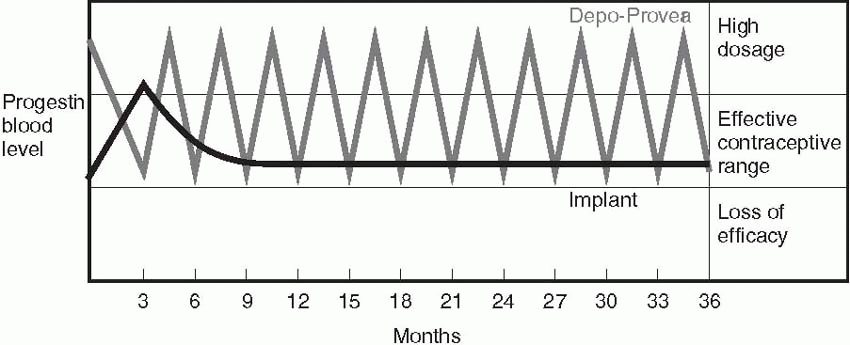
Depot-medroxyprogesterone acetate is not a “sustained-release” system; it relies on higher peaks of progestin to inhibit ovulation and thicken cervical mucus. The difference between serum levels of progestins in a sustained-release system like Implanon and a depot system like depot-medroxyprogesterone acetate is illustrated in the diagram.
Other widely used injectables are norethindrone enanthate, 200 mg every 2 months, and the monthly injectables, Lunelle (25 mg medroxyprogesterone acetate and 5 mg estradiol cypionate) and Mesigyna (50 mg norethindrone enanthate and 5 mg estradiol valerate).
 |
Mechanism of Action
The mechanism of action of depot-medroxyprogesterone acetate is different from the other lower dose, progestin-only methods because, in addition to thickening of the cervical mucus and alteration of the endometrium, the circulating level of the progestin is high enough to effectively block the luteinizing hormone (LH) surge, and, therefore, ovulation does not occur.7 Follicle-stimulating hormone (FSH) is not suppressed as it is with the combination oral contraceptive; therefore, follicular growth is maintained sufficiently to produce estrogen levels comparable to those in the early follicular phase of a normal menstrual cycle.8 Symptoms of estrogen deficiency, such as vaginal atrophy or a decrease in breast size, do not occur.
Accidental pregnancies occurring at the time of the initial injection of depot-medroxyprogesterone acetate have been reported to be associated with higher neonatal and infant mortality rates, probably due to an increased risk of intrauterine growth restriction.9,10 The timing of the first injection is, therefore, very important. To ensure effective contraception, the first injection should be administered within the first 5 days of the menstrual cycle (before a dominant follicle emerges), or a backup method is necessary for 7 days.4,11,12,13 The Quick Start, same-day start protocol can be used with depot-medroxyprogesterone acetate, with special care to rule out pregnancy and providing a backup method for 7 days.14 Same-day starts with depot-medroxyprogesterone acetate produce better continuation rates with fewer unintended pregnancies.15
The duration of action can be shortened if attention is not paid to proper administration. The intramuscular injection must be given deeply by the Z-track technique and not massaged. It is prudent to avoid locations at risk for massage by daily activities.
When given properly, there is an effective 2-week grace period that allows for late reinjections; a study of women arriving late for reinjections concluded that even a 4-week late reinjection provided equivalent protection against pregnancy.16 Women who are more than 4 weeks late for reinjection should be tested for pregnancy, reinjected if the test is negative, and advised to use backup contraception for 7 days.
Efficacy
The efficacy of this method (in both the intramuscular and subcutaneous formulations) is slightly better than that of sterilization and better than that of all the other temporary methods.4,5,6,17,18,19 In a comparison of the intramuscular and subcutaneous methods, the blood levels of medroxyprogesterone acetate are approximately 30% lower with subcutaneous administration of the lower dose, but efficacy is not impaired.4 Because serum concentrations are relatively high, efficacy is not influenced by weight (making this method a good choice for overweight women) or by the use of medications that stimulate hepatic enzymes.4,5
| ||||||||||||||||||||||||||||||||||||||||||||||||
Indications
1. At least 1 year of birth spacing desired.
2. Highly effective long-acting contraception not linked to coitus.
3. Private, coitally independent method desired.
4. Estrogen-free contraception needed.
5. Breastfeeding.
6. Sickle cell disease.
7. Seizure disorder.
Absolute Contraindications
1. Pregnancy.
2. Unexplained genital bleeding.
3. Severe coagulation disorders.
4. Previous sex steroid-induced liver adenoma.
Relative Contraindications
1. Liver disease.
2. Severe cardiovascular disease.
3. Rapid return to fertility desired.
4. Difficulty with injections.
5. Severe depression.
Advantages
Like sustained-release forms of contraception, depot-medroxyprogesterone acetate is not associated with compliance problems and is not related to the coital event. Continuation rates are better and repeat pregnancy rates are reduced compared with oral contraceptive use in teenagers; however, continuation and repeat pregnancy rates are similar when adolescents begin these methods in the immediate postpartum period.22,23 Depot-medroxyprogesterone acetate is useful for women whose ability to remember contraceptive requirements is limited. It should be considered for women who lead disorganized lives or who are mentally retarded.
The freedom from the side effects of estrogen allows depot-medroxyprogesterone acetate to be considered for patients with congenital heart disease, sickle cell anemia, patients with a previous history of thromboembolism, and women over age 30 who smoke or have other risk factors such as hypertension or diabetes mellitus. The absolute safety in regard to thrombosis is mainly theoretical; it has not been proven in a controlled study. However, an increased risk of thrombosis has not been observed in epidemiologic evaluation of depot-medroxyprogesterone acetate users, and a World Health Organization (WHO) case-control study could find no evidence for increased risks of stroke, myocardial infarction, or venous thromboembolism.3,24
Two case-control studies, one using data from the WHO Collaborative Study and one using the data from the U.K. general practice research database, assessed the risk of idiopathic venous thrombosis in users of progestins alone for therapeutic purposes, not for contraception, and concluded that therapeutic progestins alone may be associated with an increased risk of venous thromboembolism.25,26 These epidemiologic conclusions were based on extremely small numbers (only three cases in one report and five in the other) and had very wide confidence intervals. Patients who receive progestin-only for therapeutic reasons are probably older and are more likely to have family histories of cardiovascular disease. In addition, a problem of preferential prescribing is present in that clinicians are more likely to promote the use of progestin-only for women they perceive to be at greater risk of venous thromboembolism. Thus, it is likely that the case groups
represented a higher risk group than the control groups in these reports. For these reasons, we do not believe progestins are associated with an increased risk of venous thromboembolism.
represented a higher risk group than the control groups in these reports. For these reasons, we do not believe progestins are associated with an increased risk of venous thromboembolism.
An important advantage exists for patients with sickle cell disease because evidence indicates an inhibition of in vivo sickling with hematologic improvement during treatment.27 Both the frequency and the intensity of painful sickle cell crises are reduced.28
Another advantage is the finding that depot-medroxyprogesterone acetate increases the quantity of milk in nursing mothers, a direct contrast to the effect seen with estrogen-pregestin oral contraception. The concentration of the drug in the breast milk is negligible, and no effects of the drug on infant growth and development have been observed.29,30,31 In a careful study of male infants being breastfed by women treated with depot-medroxyprogesterone acetate, no metabolites of depot-medroxyprogesterone acetate could be detected in the infant’s urine and no alterations could be observed in the infant levels of FSH, LH, testosterone, and cortisol.32 Because of the slight positive impact on lactation, depot-medroxyprogesterone acetate can be administered immediately after delivery. A study to investigate the impact of early initiation found no adverse effects on breastfeeding.33 In breastfeeding, overweight, Latina women with prior gestational diabetes, the progestin-only oral minipill was associated with a 3-fold increased risk of non-insulin-dependent diabetes mellitus.34 In a similar cohort of Hispanic women, depot-medroxyprogesterone acetate was associated with a small increase in subsequent diabetes mellitus that was not statistically significant, a risk that was even lower and less significant when adjusted for the higher body weights and a greater prevalence of family history for diabetes in the users of injected contraception.35 When compared with oral contraceptive use in Navajo women, depot-medroxyprogesterone users were more likely to gain weight and develop diabetes mellitus36,37 It is possible that overweight women who already have significant insulin resistance become overtly diabetic by the added effect of progestins in a low-estrogen environment (lactation) or in an induced low-estrogen state (depot-medroxyprogesterone acetate). However, it is likely that the independent contribution of excess body weight is the more critical factor.
Depot-medroxyprogesterone acetate is an excellent contraceptive choice for women taking antiepileptic drugs because the high progestin levels raise the seizure threshold.38 An improvement in seizure control can be achieved probably because of the sedative properties of progestins.38
Women who are anticoagulated or who have bleeding disorders are prone to develop heavy menstrual bleeding and hemorrhagic ovarian cysts. Experience with depot-medroxyprogesterone acetate in these patients is limited; however, we would expect a beneficial reduction in bleeding and a reduced risk of ovarian hemorrhage, especially from a corpus luteum.39
Other benefits associated with depot-medroxyprogesterone acetate use include a decreased risk of endometrial cancer comparable with that observed
with oral contraceptives40 and probably the same benefits associated with the progestin impact of oral contraceptives: reduced menstrual flow and anemia, less pelvic inflammatory disease (PID), less endometriosis, fewer uterine fibroids,41 and fewer ectopic pregnancies. A failure to document a reduced risk of ovarian cancer by the WHO probably reflects the study’s low statistical power and the high parity in the depot-medroxyprogesterone acetate users.42
with oral contraceptives40 and probably the same benefits associated with the progestin impact of oral contraceptives: reduced menstrual flow and anemia, less pelvic inflammatory disease (PID), less endometriosis, fewer uterine fibroids,41 and fewer ectopic pregnancies. A failure to document a reduced risk of ovarian cancer by the WHO probably reflects the study’s low statistical power and the high parity in the depot-medroxyprogesterone acetate users.42
Depot-medroxyprogesterone acetate, like oral contraception, may reduce the risk of PID; however, the only study was hampered by small numbers.43 Suppression of ovulation means that ectopic pregnancies are abolished and ovarian cysts are rare.
The greater the number of choices that women have, the more likely they are to find a contraceptive that works well for them. For some women, the primary advantages of depot-medroxyprogesterone acetate are privacy and ease of use. No one but the user need know about the injection, and the 3-month schedule can be easy to maintain for women who do not mind injections. In some societies, injections are respected as efficacious, and depot-medroxyprogesterone acetate is the most popular contraceptive despite bleeding changes and other side effects.
Summary of Advantages
1. Easy to use, no daily or coital action required.
2. Safe, no serious health effects.
3. Very effective, as effective as sterilization, intrauterine contraception, and implant contraception.
4. Free from estrogen-related problems.
5. Private, use not detectable.
6. Lactation enhanced.
7. Noncontraceptive benefits.
Problems with Depot-Medroxyprogesterone Acetate
Major problems with depot-medroxyprogesterone acetate are irregular menstrual bleeding, breast tenderness, weight gain, and depression.2,3 By far, the most common problem is the change in menstrual bleeding. Up to 25% of patients discontinue in the first year because of irregular bleeding.44 The bleeding is rarely heavy; in fact, hemoglobin values rise in depot-medroxyprogesterone acetate users. The incidence of irregular bleeding is 70% in the first year, and 10% thereafter. Bleeding and spotting decrease progressively with each reinjection so that after 5 years, 80% of users are amenorrheic (compared with 10% of Norplant users).45 With the subcutaneous preparation, the bleeding pattern is similar to that with the intramuscular product; 55% achieve amenorrhea at the end of the first year of treatment and 70% after 2 years.5,19,46 Irregular bleeding can be disturbing and annoying, and, for many patients, it inhibits
sexuality; therefore, most users prefer the amenorrhea that comes with prolonged use.
sexuality; therefore, most users prefer the amenorrhea that comes with prolonged use.
If necessary, breakthrough bleeding can be treated with exogenous estrogen, 1.25 mg conjugated estrogens, or 2 mg estradiol, given daily for 7 days. A nonsteroidal anti-inflammatory product given for a week is also effective, and another option is to administer an oral contraceptive for 1 to 3 months. Giving the depot-medroxyprogesterone acetate injection earlier (more frequently) does not change the bleeding pattern.47 Most women can wait for amenorrhea without treatment if they know what to expect with time. Trying to regulate breakthrough bleeding with cyclic, repeated estradiol exposure proved to be ineffective.48 Chlamydia infection of the endometrial cavity is not a cause of the irregular bleeding associated with depot-medroxyprogesterone acetate.49
In a large international study, the most common medical reasons for discontinuing depot-medroxyprogesterone acetate during the first 2 years of use were the following:3
1. Headaches—2.3%
2. Weight gain—2.1%
3. Dizziness—1.2%
4. Abdominal pain—1.1%
5. Anxiety—0.7%
In Western societies, depression, fatigue, decreased libido, and hypertension are also encountered. Whether medroxyprogesterone acetate causes these side effects is difficult to know because they are very common complaints in nonusers as well.50 When studied closely, no increase in depressive symptoms can be observed, even in women with significant complaints of depression prior to treatment.51,52
Attempts to document a greater weight gain specifically associated with depot-medroxyprogesterone acetate have had mixed results, some finding no increase and others a small increase (e.g., about 4 kg over 5 years in one study and 11 kg over 10 years in another).53,54,55,56 In an excellent large cohort study, 3-year users of depot-medroxyprogesterone acetate increased their body weight by 5.1 kg, body fat by 4.1 kg, percent body fat by 3.4% and developed an increase in central, visceral fat, all mostly in the first 18 months and in contrast to no weight gain in oral contraceptive users.57 With the subcutaneous method, an average weight gain of 1.5 kg was observed after 1 year and 4.5 kg after 3 years, changes that are comparable to the intramuscular formulation.5,19,58 In a cohort comparison study of adolescents, obese girls gained more weight (9.4 kg after 18 months) with depot-medroxyprogesterone acetate when compared with oral contraceptives or no hormonal method.59 Specific individuals and certain ethnic groups may be more susceptible to weight gain; for example, significant weight gain was reported in Navajo women using depot-medroxyprogesterone acetate.36 In a prospective cohort study, women who experienced a significant weight gain within 6 months gained an average of about 7 kg more over 3 years, thus identifying
a susceptible group of women, about 25% of depot-medroxyprogesterone acetate users.60
a susceptible group of women, about 25% of depot-medroxyprogesterone acetate users.60
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


