Infertility
Kristen P. Wright
Julia V. Johnson
Infertility affects approximately 10% to 15% of couples and is a medical problem for 2.7 million women of reproductive age in the United States. Over the past few decades, successful treatments for virtually all causes of infertility have been developed, offering hope for couples with this medical condition. Unfortunately, many states continue to offer limited insurance coverage for infertility, minimizing effective diagnosis and treatment despite evidence that providing infertility care does not increase insurance costs. It is the goal of this chapter to identify the basic cost-effective diagnostic testing for couples with infertility that can allow the couple to rapidly move on to evidence-based therapeutic options. Prompt diagnosis and effective treatment will maximize the opportunity for conception while minimizing the cost of infertility care.
Infertility is defined as 1 year of unprotected intercourse without conception. Fecundability is the chance of conception in one menstrual cycle, and per cycle fecundity is commonly used to identify the success rate of an infertility treatment. It is expected that approximately 15% to 25% of healthy young couples will conceive in a single cycle, although clearly the chance of conception drops throughout the first year without contraception, selecting out the couples with infertility. The per cycle fecundity falls below 10% after 7 cycles, and only 3% of couples conceive during the 12th cycle. Although it is reasonable to wait a year to begin the infertility evaluation for young couples with no history suggestive of reproductive disorders, an earlier workup is indicated in couples with a positive history for a fertility-lowering disease or advancing maternal age. Decreased fecundity begins for women in their mid-30s, making it reasonable to begin the diagnostic evaluation and treatment of infertility for this age group following 6 months of unsuccessful attempts at pregnancy. When one or both members of the couple have a history suggestive of a disorder potentially altering reproduction, the diagnostic testing should begin immediately. Thus, it is critical for primary care providers and obstetrician–gynecologists to consider both the history and physical examination of both members of the couple as well as advanced maternal age when deciding the time to begin the infertility evaluation.
The increased diagnostic techniques and available treatments for infertility have raised the concern that infertility is a new disorder, increased by the exposure to environmental factors or high-risk behavior. Although the advancing age of the first pregnancy in our society does increase the risk of infertility, the primary explanation for advancing infertility treatment is the increased awareness of this disorder by patients. It is now more socially acceptable for men and women to seek treatment for infertility and to expect providers to promptly proceed with diagnostic testing and effective treatment.
Evaluation: Initial Assessment
As with all medical problems, the first step in the evaluation of infertility is a thorough medical history and physical examination. Ovulatory dysfunction, tubal risk factors, uterine and cervical abnormalities, peritoneal factors, and male factors are often identified by history or physical exam at the initial visit. It is important for both members of the couple to be interviewed at this first visit.
The known causes of infertility and their incidence are listed in Table 40.1. The history should evaluate ovulatory dysfunction based on the age of menarche, cycle length, history of increased or decreased intervals between cycles, and symptoms of ovulation and hormone production such as mittelschmerz and premenstrual molimina. If ovulatory dysfunction is identified, an endocrine review of systems may assist in determining the etiology. Information regarding thyroid symptoms, androgen excess, marked weight fluctuations, and galactorrhea should be obtained. Decreased ovarian function, as indicated by shortened menstrual cycle length, new onset of irregular cycles, or hot
flashes can indicate a contributing factor to infertility. The menstrual history also may point to other causes of infertility. Worsening dysmenorrhea, intermenstrual bleeding, and menorrhagia may point to gynecologic disease.
flashes can indicate a contributing factor to infertility. The menstrual history also may point to other causes of infertility. Worsening dysmenorrhea, intermenstrual bleeding, and menorrhagia may point to gynecologic disease.
TABLE 40.1 Causes of Infertility and Their Approximate Frequencies | ||
|---|---|---|
|
Risk factors for tubal damage have been shown to be excellent predictors of tubal factor infertility. A history of sexually transmitted diseases (STDs), pelvic inflammatory disease, pelvic surgery, ruptured appendix, septic abortion, endometriosis, and ectopic pregnancy can point to a heightened risk of tubal factor infertility. A history of uterine leiomyoma or uterine and cervical surgery also may impact fertility. Abdominal and pelvic exams can identify pelvic masses, cul-de-sac nodularity, irregular uterine contour, and fixed pelvic structures that are suggestive of tubal damage or peritoneal disease.
The history from the male partner is crucial, as sperm abnormalities account for 30% to 40% of infertility. STDs and other genitourinary infections, chemotherapy or radiation therapy, mumps during adolescence, testicular surgery or injury, and decreased ejaculatory function may herald male factor infertility. Chronic occupational exposure to extreme heat may alter sperm motility, while exposure to gametotoxic chemicals, such as nematocide dibromochloropropane, may affect sperm production. Several medications, including sulfasalazine; ketoconazole; alkylating agents such as cyclophosphamide and chlorambucil; and antiandrogens such as flutamide, cimetidine, cyproterone acetate, and spironolactone, are associated with decreased sperm production. Similarly, use of anabolic steroids for athletic performance can lead to decreased spermatogenesis. Cystic fibrosis (CF) is associated with bilateral absence of the vas deferens. An examination of the male partner, either by the obstetrician–gynecologist or an andrologist, is critical. Abnormal body habitus, lack of testicular descent, penile abnormalities, diminished size or abnormal consistency of the testes, and the presence of a varicocele may help to explain infertility.
Medical and family histories from both partners are important to identify factors that may complicate pregnancy. Preconceptual counseling typically is recommended for women with a history of diabetes, hypertension, obesity, heart disease, autoimmune diseases, thrombophilias, severe pulmonary disease, breast or gynecologic cancer, and infectious diseases such as HIV and hepatitis. Medications associated with fetal malformations, such as isotretinoin for severe cystic acne, should be discontinued. Carrier screening can be offered for inherited disorders such as CF, Tay–Sachs, thalassemia, and sickle cell anemia, based on the risk profile for each couple. A family history of genetic disorders such as fragile X and Down syndrome (trisomy 21) may indicate a need for genetic counseling. The American College of Obstetricians and Gynecologists (ACOG) now recommends offering all women prenatal diagnosis such as chorionic villus sampling or amniocentesis, and these optional tests may be discussed with women who are seeking fertility treatment. The availability of these tests should be emphasized to women over the age of 35, as they are at increased risk for conceiving a child with aneuploidy. The ACOG Technical Bulletin on preconceptional care is a valuable tool for all providers in their evaluation of couples with infertility.
The social and lifestyle history also is very important for the infertile couple. Smoking, alcohol abuse, and illicit drug use may have an adverse impact on the fertility of men and women. Smoking is particularly harmful, as it is associated with oocyte toxicity and an earlier age of menopause in women and decreased sperm motility and number in men. Excessive exercise and anorexia may adversely affect ovulation or sperm production. Exposure to potential teratogens such as lead should be excluded by the history. Although dietary alterations usually do not benefit the infertile couple, it is critical for women to begin taking at least 400 mcg of folic acid prior to conception, with many experts recommending doses up to 5 mg daily to optimally reduce the risk of neural tube defects. While moderate alcohol ingestion does not decrease fertility, women should avoid alcohol intake once they conceive. As victims of sexual and physical abuse may be markedly affected by the infertility testing and treatment required for infertility, it is important to identify these potential emotional barriers prior to the evaluation. Finally, infertility is remarkably stressful and can lead to social dysfunction or even dissolution of the couple’s relationship. One of the key members of any infertility team is the counselor or psychologist who is experienced in helping couples deal with the stress. Appropriate referrals to this individual will be discussed in the Treatment section of this chapter, but it is selectively appropriate to involve this member of team from the start of the evaluation.
A thorough physical examination should be performed on both partners. Examination of the woman should
include a complete physical exam to assess medical conditions that may affect fertility and her health during pregnancy. A bimanual exam can identify the presence of uterine enlargement, limited uterine mobility, adnexal masses, or tenderness of the pelvic organs. A speculum exam and gonorrhea and Chlamydia cultures should be performed as appropriate. Examination of the male should be performed by a physician who is experienced in male factor infertility. This examination should include a complete physical exam with assessment of penile anatomy and testicular size and careful inspection of the abdomen, inguinal region, and genitalia for surgical scars or signs of trauma. The vas deferens and epididymis should be examined for evidence of obstruction, such as epididymal induration or fullness.
include a complete physical exam to assess medical conditions that may affect fertility and her health during pregnancy. A bimanual exam can identify the presence of uterine enlargement, limited uterine mobility, adnexal masses, or tenderness of the pelvic organs. A speculum exam and gonorrhea and Chlamydia cultures should be performed as appropriate. Examination of the male should be performed by a physician who is experienced in male factor infertility. This examination should include a complete physical exam with assessment of penile anatomy and testicular size and careful inspection of the abdomen, inguinal region, and genitalia for surgical scars or signs of trauma. The vas deferens and epididymis should be examined for evidence of obstruction, such as epididymal induration or fullness.
Evaluation: Testing
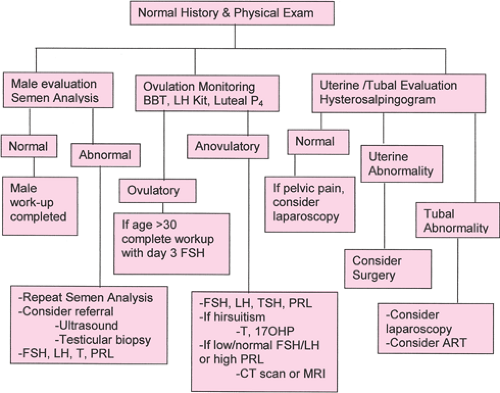
The amount of testing for most medical conditions has increased markedly over the past decade. By contrast, as our understanding of the mechanisms of infertility increases, the testing has simplified. Some traditional tests have been eliminated, as they have been shown not alter treatment decisions. The basic evaluation now includes only three tests—semen analysis, ovulation documentation, and uterine/tubal evaluation—that can be completed in 1 to 2 months. Although additional testing may be indicated in selected couples, once the cause of infertility is identified, the most cost-effective treatment can be undertaken. This lessens the couple’s stress and minimizes the cost. Studies indicate that reproductive endocrinology and infertility subspecialists have minimized their infertility evaluation and “targeted” their evaluation. This section will examine the basic evaluation, the indications for expanded evaluation, and the justification for eliminating many of the previous tests.
The semen analysis remains the primary evaluation of the male partner. This test clearly identifies subfertile men. The World Health Organization criteria in Table 40.2 are well established and were republished in 1999. The use of Kruger’s strict morphology has been associated as an improved predictor of sperm function during in vitro fertilization (IVF) cycles and is now routinely used in many centers. Some centers employ a computer-assisted semen analysis (CASA). It is important that the laboratory is experienced in the assessment of semen, but the basic analysis is satisfactory for clinical care. If the initial semen analysis is abnormal, it is important to be certain that the specimen was adequately collected, without undue stress, not exposed extremes of temperature, and not contaminated by lubricants or soaps and that there has not been a severe illness within the past 3 months that might adversely impact sperm production. A repeat semen analysis is typically obtained to confirm an abnormal semen analysis. Although it is ideal to wait 90 to 108 days to evaluate recent production of sperm, this delay in the evaluation is not generally recommended, and collection within a month is acceptable.
TABLE 40.2 Semen Analysis: Minimal Standards of World Health Organization Criteria for Normal Semen Values | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Although normal ovulation usually is assumed in women with regular menses and premenstrual moliminal symptoms, ovulation should be objectively confirmed. Only a single documented ovulation is required; there is no demonstrated value and significant frustration associated with months of repeat ovulation testing. There are three methods to evaluate ovulation. The classic method is a basal body temperature (BBT) chart. This method has a low cost, is fairly reliable, and documents the timing of ovulation. The daily BBT is obtained throughout the menstrual cycle, and the temperature should be taken first thing in the morning at the same time each day. Most women will have a temperature drop at the time ovulation, followed by a sustained temperature elevation of at least 0.4°F that coincides with the luteal phase of the menstrual cycle. It is important for the provider to evaluate this chart with the couple, as it often is difficult for them to interpret. A simpler but more costly method is the luteinizing hormone (LH) or ovulation predictor kit. Unlike the BBT chart, this method identifies the time of ovulation by identifying the LH surge 24 to 36 hours prior to release of the oocyte. This may be of value to patients whose lifestyles limit midcycle coitus. Finally, a midluteal (days 18 to 24) serum progesterone of >3 ng/mL indicates that ovulation has occurred. Although the timing of ovulation and the length of the follicular and luteal phases are not identified, this simple test unequivocally documents ovulation. Serial ultrasounds, cervical mucus examination, and endometrial biopsy also can suggest or confirm ovulation, although these tests are less frequently used because of reliability, discomfort, and cost.
For women over the age of 30, testing for decreased ovarian reserve should be added to the assessment of ovulation. The effect of advancing maternal age on fertility will be fully discussed later in the chapter, but there is no doubt that fertility begins to decrease in the mid-30s. Although the method of testing may change as our understanding of the menopausal transition increases, the classic test is a day 3 follicle-stimulating hormone (FSH) level. A normal value
for a day 3 FSH varies between laboratories, with current assays identifying decreased ovarian function with a level >10 to 15 IU/L. Although pregnancy can occur with elevated day 3 FSH levels, the chance of pregnancy is markedly reduced. Alternatively, a normal day 3 FSH should not falsely reassure women of the success of infertility treatment. This test primarily measures the number of oocytes remaining (i.e., ovarian reserve), not oocyte quality. The chance of pregnancy in the late 30s and 40s is reduced, compared with younger women, even with a normal day 3 FSH level.
for a day 3 FSH varies between laboratories, with current assays identifying decreased ovarian function with a level >10 to 15 IU/L. Although pregnancy can occur with elevated day 3 FSH levels, the chance of pregnancy is markedly reduced. Alternatively, a normal day 3 FSH should not falsely reassure women of the success of infertility treatment. This test primarily measures the number of oocytes remaining (i.e., ovarian reserve), not oocyte quality. The chance of pregnancy in the late 30s and 40s is reduced, compared with younger women, even with a normal day 3 FSH level.
Other forms of ovarian reserve testing such as the clomiphene citrate challenge test and antral follicle counts are used by some infertility specialists. The clomiphene challenge test involves measuring a day 3 FSH and estradiol level, followed by 100 mg per day of clomiphene citrate on cycle days 5 to 9 and a day 10 FSH. The clomiphene challenge test may be a better predictor of decreased ovarian reserve for older women and those with unexplained infertility. An antral follicle count utilizes a high-resolution transvaginal ultrasound on day 3 of the menstrual cycle to count the number of follicles measuring between 2 and 10 mm in diameter. A count of <3 to 6 is associated with a poorer prognosis; however, considerable cycle-to-cycle variation exists within individuals and thus a single value should be interpreted with caution.
Following the semen analysis and ovulation monitoring, the basic investigation is completed by an evaluation of the uterus and fallopian tubes. There are several options for testing, but the primary method is the hysterosalpingogram (HSG). This test is mildly uncomfortable and has a 1.4% to 3.4% risk of postprocedure infection. However, it has the benefit of visualization of both the uterine cavity and the fallopian tubes. The test is performed in the early to midfollicular phase to avoid the altered tubal function following ovulation and to prevent potential radiation exposure to an early pregnancy. Slow introduction of dye into the cervical canal below the internal os by someone skilled in the technique allows the best visualization of the uterine cavity with minimal discomfort. If any abnormality of tubal architecture is identified, antibiotics, usually doxycycline 100 mg twice a day for 5 days, are typically advised. Oil-based dye has been associated with increased pregnancy rates for a few months following the HSG, but its persistence in obstructed tubes is problematic. Water-based dye can be used initially to assure tubal patency, followed by injection of oil-based dye as a therapeutic measure. If tubal patency does not need to be assessed, the woman can undergo uterine evaluation by using ultrasound with saline injection sonohysterography or office hysteroscopy. For women with known or strongly suspected tubal damage, moving promptly to operative laparoscopy and diagnostic hysteroscopy avoids the need for the HSG. Following the basic workup, the couple will benefit from consultation with the provider. If additional testing is necessary, this evaluation and potential treatment options can be discussed with the couple. If the infertility remains unexplained, the provider may advise moving directly to treatment. In the past, all couples underwent additional male factor testing, postcoital testing, timed endometrial biopsies, and laparoscopy/hysteroscopy before the infertility evaluation was considered complete. Although this additional testing may still be advised in selected cases, most couples benefit from rapid completion of testing and prompt initiation of effective therapy. The value of advanced testing for selected patients will be considered, but the basic workup is adequate to determine effective treatment options for most couples. Figure 40.1 demonstrates the basic infertility workup followed by more advanced evaluation.
 Get Clinical Tree app for offline access 
|


