Infant Formula and Complementary Foods
Margaret Kirby
INFANT FORMULA
Infant formulas are designed to provide an acceptable substitute for human milk. Their use is indicated for (1) infants whose mothers choose not to breast-feed, (2) infants for whom human milk is contraindicated, (3) infants who require a supplement to human milk because of slow growth, and (4) infants whose mothers choose to discontinue breast-feeding before the infant is 1 year old. Despite successful efforts to increase breast-feeding to levels that exceed 90% in developing countries and 50% to 90% in industrialized countries following birth, fewer than half of infants in many countries are exclusively breast-fed by 3 to 4 months postpartum.1 Thus, infant formula provides a significant portion of the nutrient intake for many infants.
Guidelines for specific nutrient intakes by the infant intakes are detailed in Chapter 23. Infant formulas are regulated by the Food and Drug Administration in the United States2 and the European Food Safety Authority to ensure that they provide adequate nutrients at optimum bioavailability for complete nutrition for the first 4 to 6 months of infant life. 
Infant formulas commonly available in the United States are detailed in Table 25-1. These are generally available in ready-to-feed, powder, and liquid concentrate forms.  Calorie density of standard term infant formulas is about 20 calories per ounce with an osmolarity of 280 to 300 mOsm/kg, both being similar to mature human milk. Infant formulas can be categorized as either standard term infant formulas or specialized formulas. Standard term infant formulas are further categorized according to their protein type and composition: (1) cow milk based, (2) soy based, (3) hydrolyzed, and (4) elemental. Specialized formulas have altered macronutrient or electrolyte content specific for management of a medical condition.
Calorie density of standard term infant formulas is about 20 calories per ounce with an osmolarity of 280 to 300 mOsm/kg, both being similar to mature human milk. Infant formulas can be categorized as either standard term infant formulas or specialized formulas. Standard term infant formulas are further categorized according to their protein type and composition: (1) cow milk based, (2) soy based, (3) hydrolyzed, and (4) elemental. Specialized formulas have altered macronutrient or electrolyte content specific for management of a medical condition.
The American Academy of Pediatrics recommends cow milk–based, iron-fortified formula as the feeding of choice during the first year of life for infants who are not breast-fed. Unmodified cow milk is not recommended for infants in the first year of life because of the poor digestibility of fat; inadequate amounts of vitamin C, iron, zinc, and essential fatty acids; and high amounts of protein, sodium, chloride, and phosphorus that increase the potential renal solute load (see Chapter 23). Mature human milk provides a protein content of approximately 0.9 g/100 mL with 60% whey and 40% casein compared with 3.3 g/100 mL protein in cow milk with 18% whey and 82% protein.
Cow milk infant formulas are derived from cow milk with the components being reformulated or replaced to better simulate the protein, fat, carbohydrate, and mineral content of human milk. The protein content is reduced to decrease the renal solute load for the developing kidney. The protein quality is improved by adding taurine that is in much higher concentration in human milk protein than in cow milk proteins. The ratio of casein and whey milk proteins is altered to simulate the ratio in human milk. The fat content of mature human milk varies during feeding from about 3 g (foremilk) to 5 g/100 mL (hind-milk), whereas that of infant cow milk formula is 3.3 g/100 mL. Formulas are usually composed of a mixture of vegetable fats, and occasionally animal fats, to improve digestibility and absorption, increase essential fatty acid content, and provide a balance of polyunsaturated, saturated, and monounsaturated fatty acids that more closely resemble that of human milk. Standard vegetable oils used include coconut oil, providing saturated, short-chain, and medium-chain fatty acids; palm oil, providing saturated long-chain fatty acids; and soy, corn, and safflower oils, providing polyunsaturated fatty acids. Although human milk contains cholesterol, there is little or no cholesterol in cow milk–based formulas, and there is no currently known value of adding cholesterol to formulas. (See “New Additives in Infant Formula” in this section for further discussion of fatty acid composition.) The carbohydrate content of human milk is 7.3 g/100 mL compared with 4.7 g/100 mL in cow milk. The primary carbohydrate source in both milks is lactose, so additional lactose is added to cow milk to achieve a macronutrient balance similar to human milk.
Soy formulas are not recommended by the American Academy of Pediatric Committee on Nutrition except for infants whose parents have a preference for a vegetarian diet; infants with hereditary lactase deficiency (which is exceedingly rare); or infants with galactosemia.3 The American Academy of Pediatrics specifically recommends against the use of soy formulas in preterm infants, for prevention or management of colic or allergy, for infants with cow milk protein–induced enterocolitis or enteropathy, or for prevention of atopic disease in infants. Soy provides a low biological value protein and therefore protein is provided in higher amounts than in cow milk formula, with additional supplementation of methionine and taurine. The fat content of soy formula contains a blend of vegetable oils and is similar to cow milk–based formula, with additional carnitine. Lactose is avoided in soy formulas primarily because of its potential contamination with milk protein. It is replaced with sucrose, cornstarch hydrolysates, or a mixture of both. Due to the interference of soy phytates with adequate mineral absorption, most soy formulas contain increased amounts of calcium and phosphorus, zinc, and iron. Although there has been some previous concern that soy phytoestrogens affect human development, reproduction, or endocrine function, these concerns have not been substantiated.4
SPECIALIZED FORMULAS
Protein hydrolysate formula is useful in infants with cow and soy protein intolerance and for infants with malabsorption due to gastrointestinal or hepatobiliary disease. Cow milk protein is heat-treated and enzymatically hydrolyzed to yield a combination of free amino acids and peptides of varying lengths, which are then supplemented with additional amino acids to replace those lost in manufacturing. Most of these formulas are also lactose free, using tapioca starch, corn syrup solids, sucrose, or cornstarch as the carbohydrate sources. Fat blends also vary among products, with some providing medium-chain fats to aid in absorption. Elemental formulas are useful in infants with extreme protein allergies because they utilize only amino acids as the protein source. Fat and carbohydrate sources vary but are similar to the protein hydrolysate formula. Although the protein hydrolysate and elemental formulas are occasionally useful in specific clinical situations, they should not be used indiscriminately because they do have disadvantages, including: higher cost, poor taste, and often higher osmolality.
Other specialized formula and medical foods are designed to manage specific conditions. These include protein-altered formulas to treat inborn errors of metabolism, protein-altered and electrolyte-altered formulas for management of renal disease, calcium/vitamin D–altered formula for Williams syndrome, and carbohydrate-modified formulas for certain gastrointestinal disorders in which specific carbohydrates are poorly digested or absorbed. In addition, specific formulas are designed for use in preterm infants and for these infants following discharge. Recently, formulas have been marketed for the treatment of transient conditions of infancy, such as lactose-free cow milk–based formulas for postviral diarrhea, modified carbohydrate formulas (carbohydrates that thicken upon exposure to gastric acid) to limit gastroesophageal reflux, and transitional formulas with higher protein and mineral content for older infants and toddlers. The nutritional advantage of the transitional formula is uncertain.
NEW ADDITIVES
Continuing research on the optimal composition of infant milk as a substitute for human milk has recently focused on alterations in fatty acid composition and the addition of nucleotides, selenium, prebiotics, and probiotics.
Infant formulas have traditionally contained the essential fatty acids of linoleic and α-linolenic acid as the precursors to arachidonic acid and docosahexaenoic acid (DHA), which are the predominant long-chain polyunsaturated fats of the central nervous system. Some studies demonstrated improved neurodevelopmental in infants fed human milk, which contains higher concentrations of DHA than cow milk formulas, leading many formula companies to add arachidonic acid and DHA directly to formulas. This is not yet required by most regulatory bodies, but recently, recommendations to assure DHA levels between 0.2% and 0.5% of fatty acids with arachidonic acid levels at least equal to the DHA level were endorsed by several international bodies.5
Nucleotides are nitrogen-containing compounds that function as metabolic regulators, constituents of coenzymes, and sources of energy.  Currently, there is no minimum recommendation for nucleotide content of formulas, but there is a maximum level.
Currently, there is no minimum recommendation for nucleotide content of formulas, but there is a maximum level. 
The selenium concentration in human milk is much higher than in unsupplemented cow milk–based formula. This has led to selenium addition to some commercial formula.7 This may have clinical relevance for preterm infants, who have lower plasma concentrations at birth and are at higher risk of oxidative stress, although a clear benefit has yet to be demonstrated.
Prebiotics are nutrients that support the proliferation of nonpathogenic organisms, while probiotics are living bacteria. Human milk contains oligosaccharides that appear to promote the establishment of the intestinal flora (the microbiome) and therefore may have positive consequences for subsequent overall health. Probiotics are being added to infant formulas with increasing frequency. The value of probiotics should not be generalized, and each individual probiotic strain should be considered as a separate “drug.” Data regarding probiotics should also be interpreted with caution because there are no clearly established regulatory guidelines to ensure the safety and quality of commercially available probiotic preparations.
Several studies have suggested that the addition of certain oligosaccharides to infant formula can reduce the development of atopy and decrease the rate of infections during the first 2 years of life.8 Preterm infants with birth weight below 1500 g may benefit from oral probiotics through a lower incidence of necrotizing enterocolitis.9 Thus, emerging data suggest that there may be benefits for addition of prebiotics and probiotics to infant formula. The specific composition of the prebiotics and probiotics that may be beneficial at certain ages (eg, the preterm infant) or for specific disorders is the subject of intense investigation.
COMPLEMENTARY FOODS
Complementary feeding refers to the introduction of non-breast milk, non-formula foods, and liquids into the infant diet. There continue to be major differences in the timing and types of introduced complementary foods across cultures and over time, prompting ongoing research attempts to identify nutritional, growth, and health consequences of various complementary feeding patterns. The World Health Organization, the American Academy of Pediatrics, and the United Nations Children’s Fund have recommended exclusive breast-feeding for 4 to 6 months, but there is controversy regarding the exact timing of introduction of complementary foods. The American Academy of Pediatrics recommends that particularly in developing countries, where the use of potentially contaminated and/or low-nutrient dense foods puts infants at risk for diarrhea and undernutrition, infants should be exclusively breast-fed for 6 months. When these risks are lessened in developed countries, complementary foods may be introduced between ages 4 and 6 months. In infants fed an iron-fortified formula, no complementary solid foods are required before approximately 6 months to assure attainment of adequate nutrient intakes.
Complementary feeding is initiated to meet the increasing nutrient needs of growing infants, especially calories, iron, zinc, and vitamin D, and to encourage the development of feeding skills. Infants should be developmentally ready for feeding as demonstrated by the ability to sit up, to hold the head without support and by the disappearance of the extrusion reflex (pushing food out of the mouth with the tongue), and by increasing exploratory feeding behaviors. Solid foods often change the proportion of macronutrient types ingested by the infant, and vitamin and mineral intake. Complementary food usually accounts for 20% of total caloric intake at 6 months of age, and increase to 50% by 1 year of age.
Table 25-1. Common Infant Formulas Available in the United States
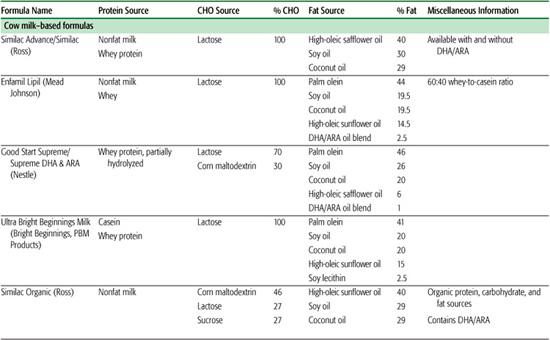
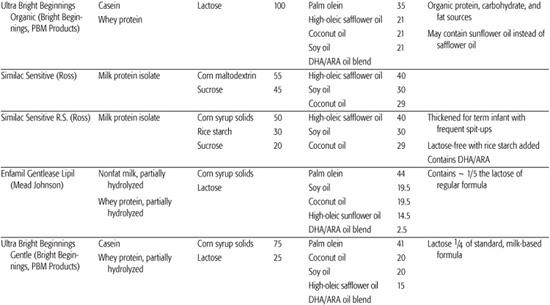
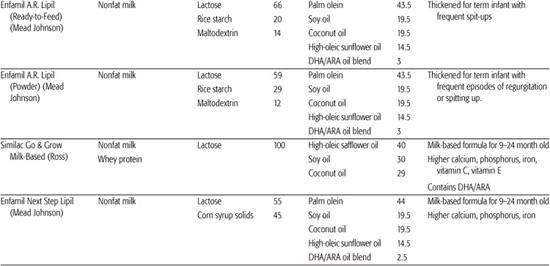
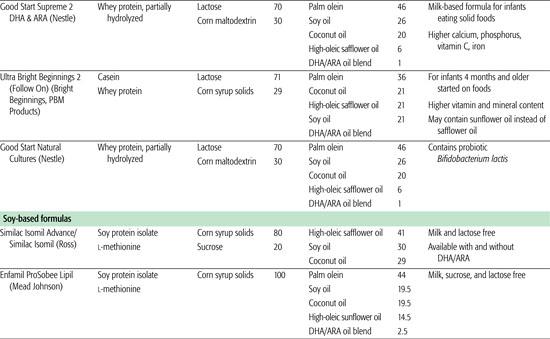
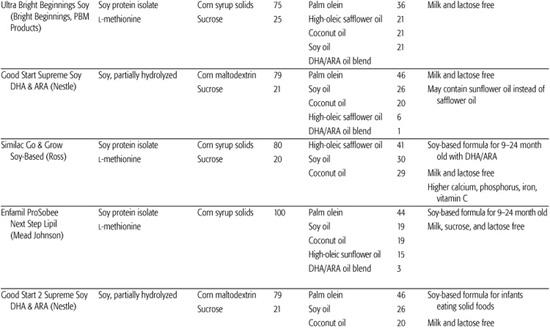
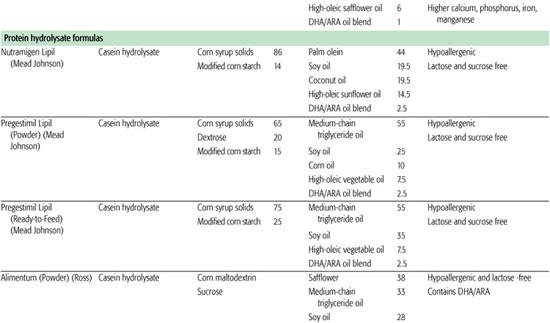
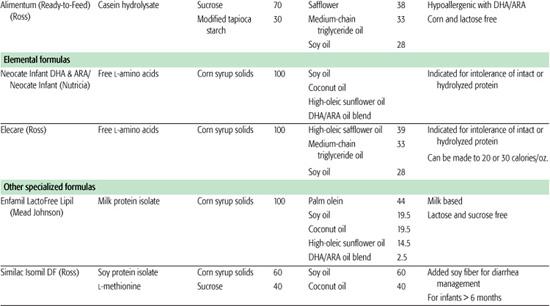
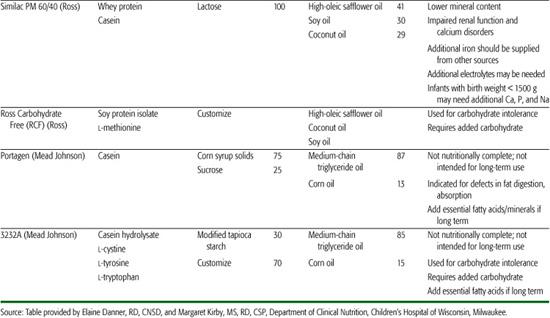
Table 25-2. Guide for Infant Feeding in the First 2 Years of Life
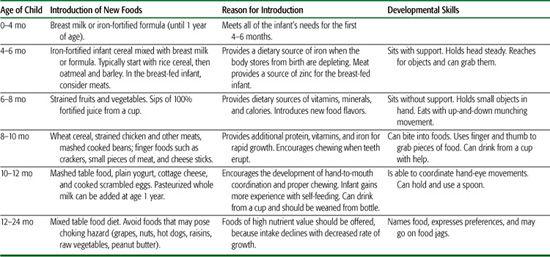
The general guidelines for introducing complementary foods are to (1) provide a safe, noncontaminated food; (2) select food for nutritional advantage; (3) use single-ingredient foods; (4) introduce 1 new food for 1 week, monitoring for allergy or intolerance as suggested by rash, wheezing, vomiting, or diarrhea; (5) initially provide as thin puree, and advance texture as developmentally appropriate, minimizing risk of aspiration; (6) avoid juice until after age 6 months, and then limit to maximum of 4 to 6 ounces a day; (7) avoid cow milk until 1 year of age (see Table 25.2).
The type of food that should first be introduced is an area of controversy. In the United States, iron-fortified rice cereal has traditionally been recommended as the first food because of the low allergenicity of rice and its fortification with iron that is required by the breast-fed infant. However, recent studies in infants support the introduction of meat as a first food for the exclusively breast-fed infant, demonstrating improved zinc levels and increased head circumference compared to infants who received iron fortified cereal as their first food.10
OTHER RECOMMENDED NUTRIENT SUPPLEMENTS
Vitamin and mineral supplement recommendations for infants include vitamin K injection at birth, vitamin D supplementation for exclusively breast-fed infants by 2 months of age, a supplemental iron source for exclusively breast-fed infants by 4 to 6 months of age, and supplemental fluoride source for all infants over 6 months of age.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


