Background
Preeclampsia is characterized by maternal endothelial dysfunction, which underlies a highly diverse clinical presentation. The pathophysiologic condition remains to be unraveled fully, but interplay between factors that are released from the placenta and maternal vascular vulnerability is likely. An imbalance in circulating angiogenic factors is a prominent feature of preeclampsia; placental growth factor and soluble Fms-like tyrosine kinase 1 have been implemented as biomarkers of placental function and preeclampsia. Their test accuracies are limited in a clinical setting, which urges better insight into their production and removal. Current data suggest that placental growth factor and soluble Fms-like tyrosine kinase 1 are released from the placenta. Both the circulating levels and the placental expression are altered in preeclamptic pregnancies. However, in vivo placental release has not been determined in human pregnancies. Moreover, there is evidence that extra-placental tissues might contribute to the circulating levels placental growth factor and soluble Fms-like tyrosine kinase 1 in normal and preeclamptic pregnancies.
Objectives
We aimed to study the in vivo placental release of placental growth factor and soluble Fms-like tyrosine kinase 1 by determining the uteroplacental arteriovenous differences in human pregnancies. Further, we investigated whether this release was altered in early-onset preeclampsia compared with control subjects and whether there was a release of placental growth factor and soluble Fms-like tyrosine kinase 1 from maternal systemic endothelium.
Study Design
We conducted a case-control study at Oslo University Hospital and included 23 women with preeclampsia (diagnosis <34 weeks) and 20 control subjects. During cesarean delivery, we sampled blood from 3 vessels simultaneously (uterine vein, radial artery, and antecubital vein). We determined concentrations of placental growth factor and soluble Fms-like tyrosine kinase 1 and calculated the arteriovenous differences. A possible net placental and extra-placental release was evaluated with the use of a Wilcoxon signed rank test. Differences between groups were compared by a Mann-Whitney U -test.
Results
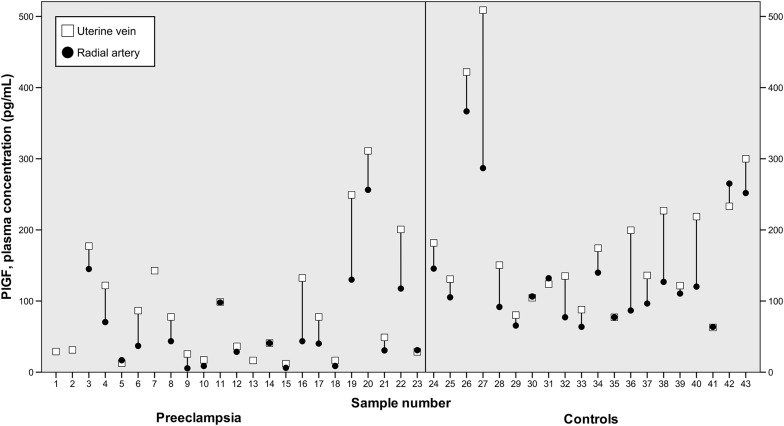
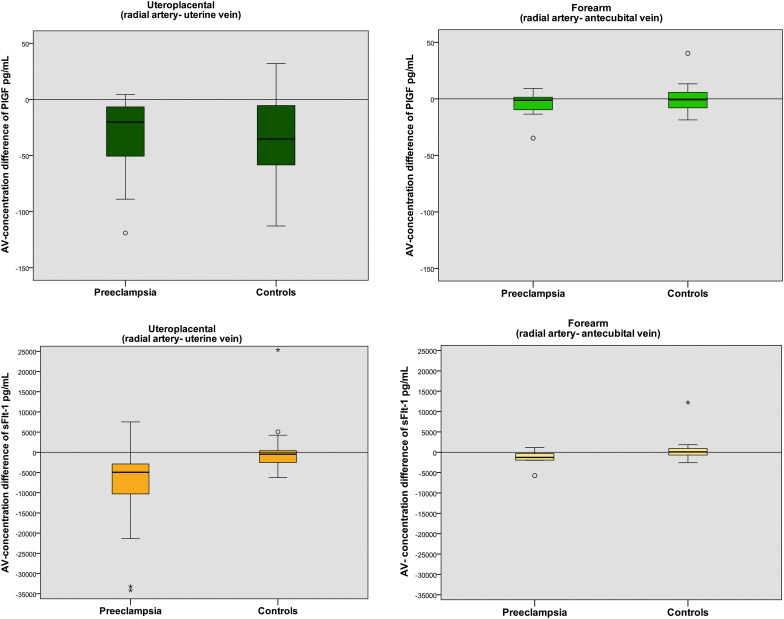
The median gestational age at delivery was 33.4 weeks (Q1, 28.3; Q3, 34.4 weeks) in the preeclamptic group and 39.3 weeks (Q1, 39.0; Q3, 39.6 weeks) in the control subjects. Women with preeclampsia had lower plasma concentrations of placental growth factor and higher concentrations of soluble Fms-like tyrosine kinase 1 compared with control subjects ( P <.001). There were significant uteroplacental arteriovenous differences of soluble Fms-like tyrosine kinase 1 in preeclampsia ( P <.001), but not in the control subjects. The uteroplacental arteriovenous differences of placental growth factor were significant in both groups ( P <.001). Despite lower concentrations of plasma placental growth factor in women with preeclampsia, the arteriovenous differences were not significantly different from normal pregnancies ( P =.53), even when we corrected for placental weight ( P =.79). We found no placental growth factor or soluble Fms-like tyrosine kinase 1 concentration differences between the radial artery and the antecubital vein.
Conclusion
Our findings are consistent with a net release of soluble Fms-like tyrosine kinase 1 from the placenta in early-onset preeclampsia. This study demonstrated a placental release of placental growth factor to the maternal circulation but could not demonstrate that this release was impaired in the preeclamptic group. We could not find evidence of systemic endothelial release of placental growth factor and soluble Fms-like tyrosine kinase 1 by analyzing the arteriovenous differences in the forearm. This study contributes to the pathophysiologic understanding of preeclampsia by the use of the clinical setting to test current concepts in vivo and underscores that studies of in vivo degradation rates of placentally released compounds are needed.
Preeclampsia is a hypertensive pregnancy complication with a diverse clinical presentation that ranges from mild hypertension and proteinuria to symptoms of severe maternal organ damage and fetal growth restriction. Preeclampsia does not develop without the presence of a placenta, but no placenta-dependent factors or pathologic features are associated consistently and specifically with the syndrome. This underscores the diversity of the syndrome and indicates that maternal predisposition may be a contributing factor in many cases of preeclampsia. The maternal clinical symptoms reflect general endothelial dysfunction, which is thought to be induced or aggravated by placenta-dependent factors. An imbalance in maternal circulating levels of proangiogenic and antiangiogenic factors is regarded as central in the pathophysiology of preeclampsia; current concepts suggest that these factors are of placental origin.
Among the most consistent findings are decreased levels of circulating placental growth factor (PlGF) and elevated levels of soluble Fms-like tyrosine kinase-1 (sFlt-1) They have been implemented as biomarkers of placental function and preeclampsia, but their test accuracies are limited in a clinical setting, which urges better insight into their production and removal. PlGF is part of the vascular endothelial growth factor (VEGF) family, which promotes proliferation, migration, and survival in endothelial cells. sFlt-1 is a soluble form of VEGF receptor 1, which acts as an antiangiogenic factor by several means, such as by binding a fraction of VEGF and PlGF in the circulation. It is proposed that cellular stress in the syncytiotrophoblast results in an altered release of these factors and causes the antiangiogenic state in preeclampsia. The placental origin of these substances is supported by in vitro studies of placental explants and cultured trophoblasts, which demonstrates that preeclampsia is associated with increased sFlt-1 expression. However, in vitro studies of PlGF diverge and do not demonstrate conclusively reduced expression in relation to preeclampsia.
The actual in vivo placental release of sFlt-1 in normal pregnancies and patients with preeclampsia is described in only 2 smaller studies. A uteroplacental release of sFlt-1 was demonstrated only in preeclamptic pregnancies. In contrast to what was expected, the single study of PlGF did not demonstrate a uteroplacental PlGF release in either normal or preeclamptic pregnancies. These findings suggest that there are significant extra-placental sources that contribute to the circulating levels of PlGF and sFlt-1 during normal pregnancy. Importantly, PlGF and sFlt-1 expression is inducible in many other cell types, including endothelial cells and monocytes. Further, some studies indicate a possible general endothelial contribution to the elevated levels of sFlt-1 that are observed in pregnancy. Both the maternal risk factors for preeclampsia and the increased risk of later cardiovascular diseases among women with preeclampsia imply that properties of the maternal endothelium might be involved in preeclampsia.
Taken together, the current concepts of human placental and extra-placental release of angiogenic factors must be further tested in vivo. This can be determined only by measuring the arteriovenous concentration differences between the incoming and outgoing vessels on the maternal side of the placenta. As far as we know, we are the only group that has established this method in preeclampsia. In the present study, we aimed to estimate the in vivo uteroplacental release of PlGF and sFlt-1 and to test whether we could demonstrate an extra-placental release by measuring the arteriovenous difference in the forearm. We hypothesized that we would find lower uteroplacental release of PlGF and higher release of sFlt-1 in preeclampsia, compared with normal pregnancies.
Materials and Methods
We conducted a case-controlled study at Oslo University Hospital, Rikshospitalet, that included preeclamptic and healthy women (control subjects), all of whom delivered by cesarean section. From 2008 until spring 2014, women were included when diagnosed with preeclampsia. Preeclampsia was defined as hypertension (systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg) and proteinuria (urine protein/creatinine ratio >30 mg/mmol). Control subjects were healthy, normotensive women who were chosen randomly from a larger study of placental nutrient transfer (fall 2012 until spring 2013). Exclusion criteria were smoking, contractions before scheduled cesarean delivery, and considerable preexisting morbidity apart from preexisting hypertension in the preeclampsia group. The included women gave a written, informed consent, and the study was approved by the Ethics Board at our institution and the Regional Committee for Medical and Health Research Ethics, South-East Norway S-07174a/2419.
Maternal clinical data were collected at inclusion. Birthweight was measured by a calibrated scale. Sex-specific z -scores were used to characterize birthweight in relation to gestational age. We previously described the sampling procedure in detail. Briefly, cesarean delivery was performed under spinal anesthesia. Blood samples were obtained from the uterine vein on the anterolateral surface of the uterus before uterine incision. Simultaneously, blood was drawn from the radial artery and the antecubital vein. The blood samples were transferred immediately to ethylenediaminetetraacetic acid vacutainers (Greiner bio-one, Kremsmunster, Austria), kept on ice, and centrifuged within 30 minutes (6°C, 2500g-force, 20 minutes). The plasma was stored at –80°C.
PlGF and sFlt-1 concentrations were measured in 1 run and in duplicate by enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN). The paired samples from each patient were always run on the same plate. The reported intra- and interassay variation coefficients were 7.0% and 11.8%, respectively, for PlGF; corresponding numbers for sFlt-1 were <10%. Assuming similar blood composition in the radial and uterine artery, we calculated the uteroplacental arteriovenous difference as the concentration difference between the uterine vein and the radial artery. A concentration difference was also calculated between the antecubital vein and the radial artery. We added standard antecubital blood samples to our procedure during the last part of the study. As a consequence, we have missing samples from the antecubital vein in the preeclamptic group but almost complete samples in the control group.
Descriptive data were reported as mean values with standard deviations (SD), median values with interquartile range (Q1,Q3), or frequency with percentages, as appropriate. Comparisons between groups were performed with the use of the independent t -test, Mann-Whitney U test, chi square, or Fischer’s exact test, as appropriate. Because of skewed distributions, PlGF and sFlt-1 concentrations were reported as median values with interquartile ranges (Q1,Q3) in text, tables, and figures. The concentrations in the 3 vessels were compared by Wilcoxon signed rank test, and differences between groups were compared by Mann-Whitney U test. A 2-sided probability value of <.05 was considered significant. All analyses were performed with Statistical Package for the Social Sciences software (version 21.0; SPSS Inc, Armonk, NY).
Results
The present study includes 23 cases of preeclampsia and 20 control subjects; their demographic and clinical characteristics are presented in Table 1 . Compared with the control subjects, the women with preeclampsia were younger and of lower parity. They were delivered at an earlier gestational age, and their neonates and placentas weighed less. There were no complications connected to the sampling, but 10% of both cases and control subjects were lost because of failure to get access to the radial artery.
| Variables | Normal pregnancies (n=20) | Preeclampsia (n=23) | P value a |
|---|---|---|---|
| Maternal | |||
| Age, y b | 36±3 | 32±5 | .07 |
| Nulliparous, n (%) | 4 (20) | 14 (61) | .007 |
| Body mass index in first trimester, kg/m 2 c | 23.9 (21.3, 25.6) | 24.5 (23.2, 31.2) | .14 |
| Antihypertensive treatment, n (%) | 0 | 22 (96) | <.001 |
| Systolic blood pressure in first trimester, mm Hg b | 108 ± 13 | 126 ± 26 | .01 |
| Diastolic blood pressure in first trimester, mm Hg b | 65 ± 8 | 79 ± 16 | .002 |
| Time from admission to delivery, d c | — | 8 (3, 18) | — |
| Newborn | (n=21) | (n=28) | |
| Twins, n (%) | 1 (5) | 5 (22) | .19 |
| Gestational age at delivery, wk c | 39.3 (39.0, 39.6) | 33.4 (28.3, 34.4) | <.001 |
| Placental weight, g b , d | 671±223 | 452±292 | .009 |
| Birthweight, g b | 3442±606 | 1577±626 | <.001 |
| Small-for-gestational age <10 percentile, n (%) e | 4 (19) | 12 (42.9) | .12 |
| Large-for-gestational age >90 percentile, n (%) e | 2 (9.5) | 0 | .18 |
a Probability values were derived by independent Student t test, chi square test, or Fischer’s exact test
c Data are given as median (Q1, Q3)
d Mean includes twin placentas
The plasma concentrations of PlGF were significantly lower in women with preeclampsia compared with the control subjects ( P <.001; Table 2 ). In both cases and control subjects, the plasma concentration of PlGF was higher in the uterine vein than in the radial artery and the antecubital vein ( P ≤.001 in both groups; Figure 1 ). Consequently, we observed significant uteroplacental arteriovenous differences in both preeclamptic and normal pregnancies ( Figures 1 and 2 ). Despite lower concentrations of PlGF in women with preeclampsia, these arteriovenous differences were not significantly different between the 2 groups ( P =.53). There was no difference between the plasma concentrations in the radial artery and the antecubital vein in either group ( Figure 2 ).
| Variable | Group | |||||
|---|---|---|---|---|---|---|
| Control (n=20) | Preeclampsia (n=23) | |||||
| Uterine vein (n=20) | Radial artery (n=20) | Antecubital vein (n=19) | Uterine vein (n=23) | Radial artery (n=19) | Antecubital vein (n=10) | |
| Placental growth factor, pg/mL | 143 (109, 225) | 108 (80, 144) | 110 (84, 145) | 49 a (26, 132) | 40 a (17, 98) | 40 b (26, 130) |
| Placental growth factor/placenta weight, pg/mL/g | 0.24 (0.18, 0.36) | 0.18 (0.15, 0.24) | — | 0.15 b (0.09, 0.26) | 0.10 a (0.06, 0.16) | — |
| sFlt-1, ng/mL | 8.4 (5.4,13.2) | 7.5 (4.9,12.5) | 7.8 (4.5, 14.0) | 28.6 a (19.1, 44.8) | 18.9 a (15.3, 36.1) | 21.4 a (16.2, 31.2) |
| sFlt-1/placenta weight, pg/mL/g | 14.8 (11.3, 18.0) | 13.3 (10.0, 18.0) | — | 75.94 a (44.0, 142.98) | 51.3 a (36.0, 106.5) | — |
| sFlt-1/placental growth factor ratio | 52 (32, 83) | 77 (46, 101) | 72 (47, 90) | 467 a (203, 1747) | 427 a (182, 2118) | 347 a (150, 849) |
a P <.001, compared with control group

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


