Implant Contraception
The high rate of unintended pregnancies and the relatively high failure rates with the typical use of reversible methods of contraception are strong indications of a need for long-acting contraceptive methods that are easier to use. Implantable, subdermal capsules that release progestins for several years are a response to this need.
There are three major implant systems: Implanon, Norplant, and Jadelle (formerly called “Norplant-2”). A Chinese version of Jadelle is called Sinoplant II; both contain 150 mg levonorgestrel. Unfortunately, Norplant was withdrawn from the U.S. market in a business decision dictated by profit and liability despite the fact that it provided an excellent option for contraception. Jadelle is approved by the U.S. Food and Drug Administration, but it has not been marketed. In many parts of the world, Jadelle has replaced the use of Norplant; however, Norplant is still used worldwide.
Implanon differs from Norplant and Jadelle in many pivotal aspects, chiefly one rod instead of Norplant’s six capsules and Jadelle’s two rods, and a less androgenic progestin.1,2 Like Norplant, Implanon has been extensively marketed throughout the world with a good track record and high continuation rates. Contraceptive implants are approved in more than 60 countries and used by approximately 11 million women.2
The long-acting progestin methods are as effective as sterilization and intrauterine devices (IUDs), and more effective than oral and barrier contraception.3 An important reason for this high efficacy in actual use is the nature of the delivery systems themselves, which require little effort on the part of the user. Because compliance does not require frequent resupply or instruction in use, as with oral contraception, the actual or typical use effectiveness is very close to the theoretical (lowest expected) effectiveness.
Sustained-release methods require less of the user, but they demand more of the clinician. Implants involve minor operative procedures for placement and for discontinuation. Clinicians have a special responsibility to become skillful in the operations required to remove implants and to be available to women when those skills are required to terminate use. Disturbances of menstrual patterns and other side effects prompt many more questions from patients about these methods than about use of the familiar oral, intrauterine, and barrier contraceptives.4
Implant Systems
Norplant was developed by the Population Council and first approved in 1983 in Finland, where it was manufactured. It was approved in the United States in 1990, marketed in 1991, and withdrawn from the market in 2002.
Norplant is a “sustained-release” system using silastic tubing permeable to steroid molecules to provide stable circulating levels of synthetic progestin over years of use. The Norplant system consists of six capsules, each measuring 34 mm in length with a 2.4-mm outer diameter and containing 36 mg crystalline levonorgestrel. The capsules are made of flexible, medicalgrade silastic (polydimethylsiloxane and methylvinyl siloxane copolymer) tubing that is sealed shut with silastic medical adhesive (polydimethylsiloxane). The six capsules contain a total of 216 mg levonorgestrel, which is very stable and remained unchanged in capsules examined after more than 9 years of use.
Jadelle was also developed by the Population Council and manufactured in Finland. It was approved in the United States in 1996, but never marketed. The thin, flexible Jadelle rods are wrapped in silastic tubing (the same material used by Norplant), 43 mm in length and 2.5 mm in diameter, thus slightly longer and thicker than Norplant.5 Each rod contains 75 mg levonorgestrel for a total of 150 mg, 66 mg less than that in the six Norplant capsules. Whereas the levonorgestrel in Norplant is packed into the capsules in crystal form, the core of the Jadelle rod is a mixture of levonorgestrel and an elastic polymer (dimethylsiloxane/methylvinylsiloxane). Long-term clinical trials indicate that the performance and side effects are similar to Norplant, but removal is faster.6,7
Norplant average release rate
First month—85 µg levonorgestrel daily
After 1 year—35 µg
After 2 years—30 µg
Jadelle average release rate
First month—100 µg levonorgestrel daily
After 1 year—40 µg
After 2 years—30 µg
Because the release rates with the two levonorgestrel systems are comparable, it is reasonable to conclude that clinical studies with Norplant and Jadelle should yield similar results. In the discussion that follows, the morestudied product, Norplant, is often cited, but clinicians can assume that the findings apply as well to Jadelle.
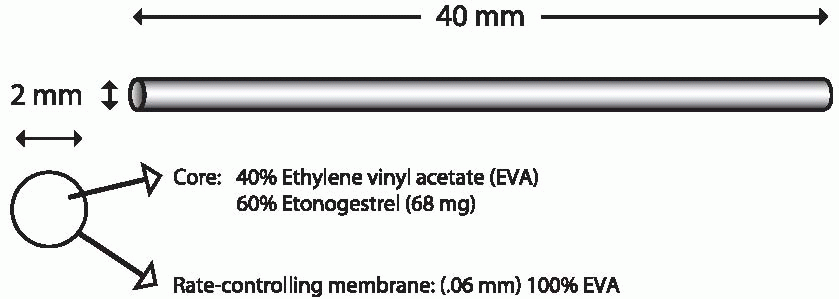
Implanon is a single flexible rod, 4 cm long and 2 mm in diameter, that contains 68 mg of 3-keto desogestrel (etonogestrel, the active metabolite of desogestrel) dispersed in a core of ethylene vinyl acetate wrapped with
a 0.6-mm thick membrane of the same material. There is no evidence that either ethylene vinyl acetate or silastic has toxic effects when implanted.8 The hormone is released at an initial rate of about 67 µg/d decreasing to 30 µg after 2 years; concentrations that inhibit ovulation are achieved within 8 hours of insertion.9 A steady state is achieved after 4 months, after which there is a gradual decline.9 Implanon, placed subdermally with a disposable inserter, suppresses ovulation for 2.5 years and provides effective contraception for at least 3 years. Side effects are similar to those with Norplant or Jadelle, except for less bleeding and a higher rate of amenorrhea with Implanon.10,11,12,13
a 0.6-mm thick membrane of the same material. There is no evidence that either ethylene vinyl acetate or silastic has toxic effects when implanted.8 The hormone is released at an initial rate of about 67 µg/d decreasing to 30 µg after 2 years; concentrations that inhibit ovulation are achieved within 8 hours of insertion.9 A steady state is achieved after 4 months, after which there is a gradual decline.9 Implanon, placed subdermally with a disposable inserter, suppresses ovulation for 2.5 years and provides effective contraception for at least 3 years. Side effects are similar to those with Norplant or Jadelle, except for less bleeding and a higher rate of amenorrhea with Implanon.10,11,12,13
Indications
Contraceptive implants are a good choice for women of reproductive age who are sexually active and desire long-term, continuous contraception. Implants should be considered for women who
1. Want to delay the next pregnancy for at least 2 to 3 years.
2. Desire a highly effective, long-term method of contraception.
3. Experience serious or minor estrogen-related side effects with estrogen-progestin contraception.
4. Have difficulty remembering to take pills every day, have contraindications or difficulty using IUDs, or desire a non-coitus-related method of contraception.
5. Have completed their childbearing but are not yet ready to undergo permanent sterilization.
6. Have a history of anemia with heavy menstrual bleeding.
7. Intend to breastfeed for a year or 2.
8. Have chronic illnesses, in which health will be threatened by pregnancy.
Absolute Contraindications
Implant use is contraindicated in women who have
1. ACTIVE thrombophlebitis or thromboembolic disease.
2. Undiagnosed genital bleeding.
3. ACUTE liver disease.
4. Benign or malignant liver tumors.
5. Known or suspected breast cancer.
Relative Contraindications
Based on clinical judgment and appropriate medical management, implants MAY BE USED by women with a history of or current diagnosis of the following conditions:
1. Heavy cigarette smoking (15 or more daily) in women older than 35 years.
2. History of ectopic pregnancy.
3. Diabetes mellitus. Because multiple studies have failed to observe a significant impact on carbohydrate metabolism, implants, in our view, are particularly well suited for diabetic women.
4. Hypercholesterolemia.
5. Hypertension.
6. History of cardiovascular disease, including myocardial infarction, cerebral vascular accident, coronary artery disease, angina, or a previous thromboembolic event. Patients with artificial heart valves.
7. Gallbladder disease.
8. Chronic disease, such as immunocompromised patients.
Implants are not contraindicated in the following situations, but other methods are preferable:
1. Severe acne.
2. Severe vascular or migraine headaches.
3. Severe depression.
4. Concomitant use of medications that induce microsomal liver enzymes:
Carbamazepine (Tegretol)
Felbamate
Lamotrigine
Nevirapine
Oxcarbazepine
Phenobarbital
Phenytoin (Dilantin)
Primidone (Mysoline)
Rifabutin
Rifampicin (Rifampin)
St. John’s wort
Topiramate
Vigabatrin
Possibly valproic acid, ethosuximide, griseofulvin, and troglitazone
Mechanism of Action
The release rate of the contraceptive implants is determined by total surface area and the density of the implant in which the progestin is contained. The progestin diffuses from the implant into the surrounding tissues where it is absorbed by the circulatory system and distributed systemically, avoiding an initial high level in the circulation as with oral or injected steroids. Within 8 hours after insertion of Implanon, plasma concentrations of etonogestrel are about 300 ng/mL, high enough to prevent ovulation.16 A study of cervical mucus changes with Norplant indicates that a backup method should be used for 3 days after insertion of Norplant or Jadelle; this is not necessary when Implanon is inserted as directed.17,18 Progestin concentrations are much more variable with Norplant and Jadelle than with Implanon.16
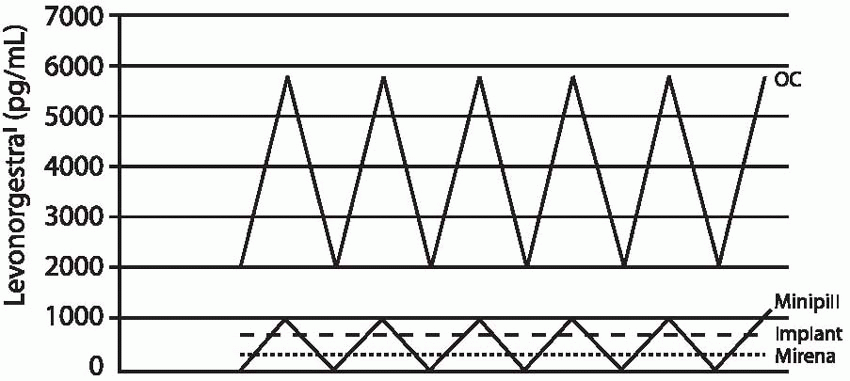 |
The Implanon rod releases 60 µg of etonogestrel per 24 hours at 3 months of use. This rate declines gradually to 40 to 50 µg daily by 12 months and 30 µg/d by 2 years of use. The 85 µg of hormone released by Norplant or the 100 µg released by Jadelle during the first few months of use is about equivalent to the daily dose of levonorgestrel delivered by the progestin-only, minipill oral contraceptive, and 25% to 50% of the dose delivered by lowdose combined oral contraceptives. After 6 months of use, daily levonorgestrel concentrations are about 0.35 ng/mL; at 2.5 years, the levels decrease to 0.25 to 0.35 ng/mL. Until the 8-year mark, mean levels remain above 0.25 ng/mL.19 Mean plasma concentrations below 0.2 ng/mL are associated with increased pregnancy rates for Norplant (lower levels are more likely in heavier women).
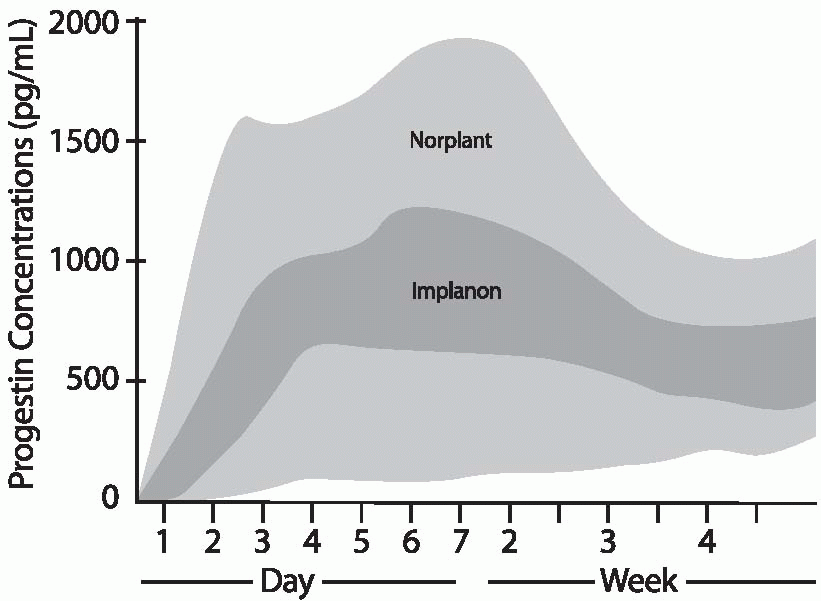 |
Body weight affects the circulating levels of levonorgestrel; the greater the weight of the user, the lower the levonorgestrel concentrations at any time during Norplant or Jadelle use. The greatest decrease over time occurs in women weighing more than 70 kg (154 lb), but even for heavy women, the release rate is high enough to prevent pregnancy at least as reliably as oral contraceptives. In Implanon users, etonogestrel concentrations are affected very little by body weight, and failure rates did not increase with increasing body weight in the small numbers of overweight women in the clinical trials.20 Although the data with overweight women are limited, it is likely that Implanon is a good contraceptive choice for obese women.
Levonorgestrel levels can also be affected by the circulating levels of sex hormone-binding globulin (SHBG). Levonorgestrel has a higher affinity for SHBG than does etonogestrel. In the week after Norplant or Jadelle insertion, SHBG levels decline rapidly and then return to approximately half of preinsertion levels by 1 year of use. This effect on SHBG is not uniform and may account for some of the individual variations in circulating progestin concentrations.21
Implants are highly effective contraceptives. There are three probable modes of action, which are similar to those attributed to the contraceptive effect of the progestin-only minipills, but because daily dosing is not required, implants are more effective than oral methods.
1. The progestin suppresses, at both the hypothalamus and the pituitary, the luteinizing hormone (LH) surge necessary for ovulation. As determined by progesterone levels in many users over several years, approximately one third of all cycles in Norplant users are ovulatory.19,22 During the first 2 years of use, only about 10% of women
are ovulatory, but by 5 years of use, more than 50% are. In those cycles that are ovulatory, there is a high incidence of luteal insufficiency. Implanon inhibits ovulation throughout a 3-year period, accounting for almost all of the contraceptive effect.2 However, follicular development does occur, avoiding the problem of clinically significant hypoestrogenemia, and in the last 6 months of the 3-year period with Implanon, there is an occasional ovulation.16,23
are ovulatory, but by 5 years of use, more than 50% are. In those cycles that are ovulatory, there is a high incidence of luteal insufficiency. Implanon inhibits ovulation throughout a 3-year period, accounting for almost all of the contraceptive effect.2 However, follicular development does occur, avoiding the problem of clinically significant hypoestrogenemia, and in the last 6 months of the 3-year period with Implanon, there is an occasional ovulation.16,23
2. The steady release of progestin has a prolonged effect on the cervical mucus. The mucus thickens and decreases in amount, forming a barrier to sperm penetration.17,23,24,25
3. The progestin suppresses the estradiol-induced cyclic maturation of the endometrium and eventually causes atrophy. These changes could prevent implantation should fertilization occur; however, no evidence of fertilization can be detected in Norplant users.26
Advantages
Implants are a safe, highly effective, continuous method of contraception that requires little user effort and, unlike long-acting injectable contraception, is rapidly reversible. Because this is a progestin-only method, it can be used by women who have contraindications for the use of estrogen-containing contraceptives. The sustained release of low doses of progestin avoids the high initial dose delivered by injectables and the daily hormone surge associated with oral contraceptives. Implants are an excellent choice for a breastfeeding woman and can be inserted immediately postpartum. There are no effects on breast milk quality or quantity, and infants grow normally.27,28,29 Another advantage of the implant method is that it allows women to plan their pregnancies precisely; return of fertility occurs within a few weeks, in contrast to the 6- to 18-month delay in ovulation that can follow depot-medroxyprogesterone acetate injections.16,30,31,32
One of the major advantages of sustained-release methods is the high degree of efficacy, nearly equivalent to the theoretical effectiveness. In couples for whom elective abortion is unacceptable in the event of an unplanned pregnancy, the high efficacy rate is especially important. There are no forgotten pills, broken condoms, lost diaphragms, or missed injections. For women who are at high risk of medical complications should they become pregnant, sustained-release implants present a significant safety advantage. Users should be reassured that implant use has not been associated with changes in carbohydrate or lipid metabolism, coagulation, liver or kidney function, or immunoglobulin levels. Because many women wanting implants will have had negative experiences with other contraceptives, it is important that the differences between this method and previous methods be explained.
Disadvantages
There are some disadvantages associated with the use of the implant systems. Implants cause disruption of bleeding patterns, especially during the first year of use, and some women or their partners find these changes unacceptable.4 Endogenous estrogen is nearly normal, and unlike the estrogen-progestin contraceptives, progestin is not regularly withdrawn to allow endometrial sloughing. Consequently, the endometrium sheds at unpredictable intervals.
The implants must be inserted and removed in a surgical procedure performed by trained personnel. Women cannot initiate or discontinue the method without the assistance of a clinician. The incidence of complicated removals is approximately 5% for Norplant or Jadelle and lower for Implanon, an incidence that can be best minimized by good training and careful insertion.38,39 The implants can be visible under the skin. This sign of the use of contraception may be unacceptable for some women and for some partners.4
Implants do not provide protection against sexually transmitted infections (STIs) such as herpes, human papillomavirus, HIV, gonorrhea, or chlamydia. Although users may be less likely to use a second method because of the high contraceptive efficacy,40 users at risk for STIs must use condoms as a second method to prevent infection.
Because the insertion and removal of implants require minor surgical procedures, initiation and discontinuation costs are higher than with oral contraceptives or barrier methods. The cost of implants plus fees for insertion total an amount that may seem high to patients unless they compare it with the total cost of using other methods for up to 5 years.41 Nevertheless, short-term use is expensive compared with the relatively low initial costs of other reversible methods, and most women cannot be expected to use longacting methods for their full duration of action.
Cultural factors can influence the acceptability of menstrual changes. Some cultures restrict a woman from participating in religious activity, household activities, or sexual intercourse while menstruating. All users must be aware of the possible menstrual changes. It is important to stress that all of the menstrual changes are expected, that they do not cause or represent illness, and that most women revert back to a more normal pattern with increasing duration of use.
Insertion and removal of implants will be a new experience for most women. As with any new experience, women will approach it with varying degrees of apprehension and anxiety. In reality, most patients are able to watch in comfort as implants are inserted or removed. Women should be told that the incisions used for the procedures are very small and heal quickly, leaving small scars that are usually difficult to see because of their location and size.
We encourage prospective users to see and touch implants. Women can be reassured that the implants will not be damaged or move if the skin above them is accidentally injured. Normal activity cannot damage or displace the implants. Most women become unaware of their presence. A few women report sensing the implants if they have been touched or manipulated for a prolonged period of time, or after vigorous exercise. The implants are more visible in slender
women with good muscle tone. Darker-skinned users may notice further darkening of the skin directly over the implants; this resolves after removal.
women with good muscle tone. Darker-skinned users may notice further darkening of the skin directly over the implants; this resolves after removal.
Efficacy
Contraceptive implants provide highly effective birth control. In 2- or 3-year studies in 11 international clinical trials of 942 women using Implanon, no pregnancies occurred.20 In studies of Norplant conducted in 11 countries, totaling 12,133 woman-years of use, the pregnancy rate was 0.2 pregnancies per 100 woman-years of use.15,30 All but one of the pregnancies that occurred during the Norplant evaluation were present at the time of implant insertion. If these luteal phase insertions are excluded from analysis, the first-year pregnancy rate was 0.01 per 100 woman-years. In adolescents, Norplant implants provide better protection against unwanted pregnancy, compared with oral contraceptives, and an important factor is the better continuation rate with Norplant.40,42,43
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
There are no weight restrictions for Norplant or Jadelle users, but heavier women (more than 70 kg) may experience slightly higher pregnancy rates in the later years of use compared with lighter women. Even in the later years, however, pregnancy rates for heavier women using Norplant are lower than with oral contraception. The differences in pregnancy rates by weight are probably due to the dilutional effect of larger body size on the low, sustained serum levels of levonorgestrel. Heavier women should not rely on Norplant or Jadelle beyond the 5-year limit. For slender women, the duration of efficacy extends well past the fifth year of use. In some extended trials, no pregnancies have occurred into the seventh year. Data are not available regarding the effect of body weight on the efficacy of Implanon, but unlike Norplant and Jadelle, progestin levels are not significantly lower in heavier women.
The contraceptive efficacy of Implanon surpasses that of Norplant and sterilization.2 Only a rare pregnancy occurs, resulting in a Pearl Index of about 0.01.23,46 In over 70,000 cycles, no pregnancies were recorded because of total inhibition of ovulation until ovulations were observed in the last 6 months of the 3-year period.23,47 Postmarketing surveillance of pregnancies in Australia, where nearly one quarter of contraceptors relied on Implanon in 2004, revealed that of 218 pregnancies, only 13 could possibly have been failures of the method.48 In Australia and the Netherlands, pregnancies commonly were the consequence of poor insertion technique, especially allowing the implant to fall unnoticed to the floor.
Implants have an immediate contraceptive effect when inserted within the first 7 days of a menstrual cycle, but when insertion is after day 7, a backup method of contraception is necessary for at least 3 days.49
Ectopic Pregnancy
The ectopic pregnancy rate during Norplant use is 0.28 per 1,000 womanyears. 15 Although the risk of developing an ectopic pregnancy during use of Norplant is low, when pregnancy does occur, ectopic pregnancy should be suspected because approximately 30% of Norplant pregnancies are ectopic. Because Implanon is more effective in inhibiting ovulation, we would expect the risk of ectopic pregnancy to be considerably less than that associated with Norplant.
Menstrual Effects
Menstrual bleeding patterns are highly variable among users of implant contraception. With levonorgestrel implants, some alteration of menstrual patterns will occur during the first year of use in approximately 80% of
users, later decreasing to about 40%, and by the fifth year, to about 33%.52,53 The changes include alterations in the interval between bleeding, the duration and volume of menstrual flow, and spotting. Oligomenorrhea and amenorrhea also occur but are less common, less than 10% after the first year and diminishing thereafter. Irregular and prolonged bleeding usually occurs during the first year. Although bleeding problems occur much less frequently after the second year, they can occur at any time.53,54 Studies of the endometrium in Norplant users experiencing abnormal bleeding indicate the presence of enlarged venous sinusoids (fragile vessels) and a reduction in the expression of a protein factor (perivascular stromal cell tissue factor) involved in the initiation of hemostasis.55 Within weeks after insertion, the density of endometrial small blood vessels increases and the endometrium regresses to an atrophic state.56 It is believed that bleeding is a consequence of rapid endometrial regression and that the apparent increase in the number of blood vessels may reflect increased tortuosity accompanying the atrophic regression.
users, later decreasing to about 40%, and by the fifth year, to about 33%.52,53 The changes include alterations in the interval between bleeding, the duration and volume of menstrual flow, and spotting. Oligomenorrhea and amenorrhea also occur but are less common, less than 10% after the first year and diminishing thereafter. Irregular and prolonged bleeding usually occurs during the first year. Although bleeding problems occur much less frequently after the second year, they can occur at any time.53,54 Studies of the endometrium in Norplant users experiencing abnormal bleeding indicate the presence of enlarged venous sinusoids (fragile vessels) and a reduction in the expression of a protein factor (perivascular stromal cell tissue factor) involved in the initiation of hemostasis.55 Within weeks after insertion, the density of endometrial small blood vessels increases and the endometrium regresses to an atrophic state.56 It is believed that bleeding is a consequence of rapid endometrial regression and that the apparent increase in the number of blood vessels may reflect increased tortuosity accompanying the atrophic regression.
Implanon alters menstrual patterns, but amenorrhea occurs more often (21% of users in the first year, 30% to 40% after 1 year) than with Norplant.11,13 A single Implanon rod completely suppresses ovulation for 2.5 years, and, therefore, menses do not become more regular after the first 2 years as with Norplant. After 2 years, ovulation occurs in about half of the menstrual cycles. Bleeding is lighter and less frequent among Implanon users because more profound ovarian suppression results in less follicular estrogen production and less endometrial stimulation; nevertheless, irregular bleeding continues to be a major reason for discontinuation.13,57
Despite an increase in the number of spotting and bleeding days over preinsertion menstrual patterns, hemoglobin concentrations rise in Norplant users because of a decrease in the average amount of menstrual blood loss.58,59,60,61 Implanon likewise does not cause anemia.11
Implant users who can no longer tolerate prolonged bleeding will benefit from a short course of oral estrogen: conjugated estrogens, 1.25 mg, or estradiol, 2 mg, administered daily for 7 days.62 A therapeutic dose of one of the prostaglandin inhibitors given during the bleeding will help to diminish flow, but estrogen is the most effective treatment.63,64 Another approach is to administer an estrogen-progestin oral contraceptive for 1 to 3 months.65
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree