Immunizations
Kristina A. Bryant
Childhood immunizations represent one of the great public health achievements of the 20th and 21st centuries.1 Less than 250 years after Edward Jenner discovered that inoculation with cowpox protected against smallpox, immunization against 14 different diseases before age 2 is routinely recommended in the United States.
Vaccines can be broadly categorized as live or inactivated vaccines. Live vaccines, including vaccines against measles, mumps, rubella, varicella, and rotavirus, contain organisms that have been attenuated or weakened. They replicate in the host, simulating natural infection, but they rarely cause disease.
The immune response elicited by live attenuated vaccines is nearly identical to that produced after natural infection. Live attenuated vaccines stimulate both humoral and cell-mediated immunity. Some live attenuated vaccines are effective after a single dose and, in general, immunity after immunization with a live vaccine is long-lasting.3 Preexisting antibody to a live attenuated vaccine antigen, such a persisting transplacental maternal antibody in an infant, may interfere with replication of the vaccine virus in the vaccinee and prevent the development of an immune response. Rarely, live attenuated vaccines cause severe or fatal reactions as a result of uncontrolled replication in immunocompromised hosts.
Inactivated vaccines may be produced from killed or inactivated whole bacteria (eg, the pertussis component of diphtheria–tetanus–whole cell pertussis vaccine, or DTwP) or viruses, or purified fractions of bacteria or viruses. Fractional vaccines may be protein or polysaccharide based. Protein-based vaccines include inactivated bacterial toxins called toxoids (the basis of diphtheria and tetanus vaccines) and subvirion or subunit products (inactivated influenza vaccine).
Polysaccharide-based vaccines containing pure bacterial cell wall polysaccharide include early vaccines for the prevention of disease caused by Haemophilus influenzae type B (Hib), Streptococcus pneumoniae, and Neisseria meningitidis. Because polysaccharide antigens are T-cell-independent antigens, they are poorly immunogenic in children younger than age 2 and do not elicit immune memory in older children and adults. Conjugation of bacterial capsular polysaccharide to a protein carrier changes the immune response elicited by the vaccine antigen to a T-cell-dependent process, thus improving immunogenicity in young children and evoking immunologic memory. Polysaccharide-protein conjugate vaccines for the prevention of Hib, S pneumoniae, and N meningitidis, have also been shown to decrease nasopharyngeal carriage of vaccine-specific organisms, a phenomenon that has contributed to herd immunity.
Inactivated vaccines may also be produced by recombinant DNA technology. For example, the vaccine antigen for hepatitis B vaccine is produced by yeast cells into which a gene for hepatitis B surface antigen has been inserted.
Unlike live vaccines, inactivated vaccines do not replicate in the host and cannot cause disease. They are generally considered less reactogenic than live vaccines. However, multiple doses of vaccine are generally required to elicit immunity. Immunity wanes over time, necessitating booster doses.
National immunization policy in the United States is made by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). Recommendations for the use of vaccines are also made by professional organizations such as the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP); these are typically harmonized with the ACIP recommendations. Recommendations for the immunization of children and adolescents are updated annually (Tables 244-1 and 244-2).4 Catch-up schedules for children who start their immunizations late or who fall 1 month or more behind are detailed in Table 244-3. Three vaccines are recommended specifically for adolescents. Tetanus–diphtheria–acellular pertussis (Tdap) vaccine and meningococcal conjugate vaccine are recommended for all adolescents. Human papillomavirus (HPV) vaccine is recommended for adolescent females and may be given to adolescent males. Updated schedules can be obtained at: http://www.cdc.gov/vaccines/recs/schedules/child-schedule.htm.
To maximize efficacy and minimize toxicity, recommendations regarding the schedule, dose, route, and site of administration should be followed for each vaccine.5 Most routinely recommended childhood vaccines are administered intramuscularly or subcutaneously. Important exceptions include rotavirus vaccine, which is administered orally, and live attenuated influenza vaccine, which is administered intranasally. Manufacturer recommendations should be followed for specific vaccines. Failure to administer a vaccine according to the recommended route may result in decreased vaccine efficacy or increased adverse effects.
In general, intramuscular injections are administered in the anterolateral thigh in infants and the deltoid muscle of older children and adolescents. For toddlers, either the anterolateral thigh or the deltoid may be used. The buttock should be avoided as a site of injection because of the potential for sciatic nerve damage and inconsistent muscular deposition. Changing needles after withdrawing a vaccine dose from a vial but before administration to a patient is not necessary, nor is pulling back on the vaccine plunger after insertion of the needle but before administration.
There are no contraindications to simultaneous administration of any vaccines. In order to facilitate completion of the vaccine schedule and minimize the period during which a child is susceptible to a vaccine-preventable disease, all vaccines for which a child is eligible at a given visit should be administered. The use of a combination vaccine is generally preferred over separate injections of equivalent component vaccines. Considerations should include provider assessment, patient preference, and the potential for adverse events. Details are available in the Advisory Committee on Immunization Practices statements available at http://www.cdc.gov/vaccines/pubs/acip-list.htm. However, different vaccines should not be mixed in the same syringe unless specifically licensed for use in this manner. “Split” or partial doses of vaccines should never be administered, as this may result in decreased immunogenicity.
Table 244–1. Recommended Immunization Schedule for Persons Aged 0 Through 6 Years—United States, 2010
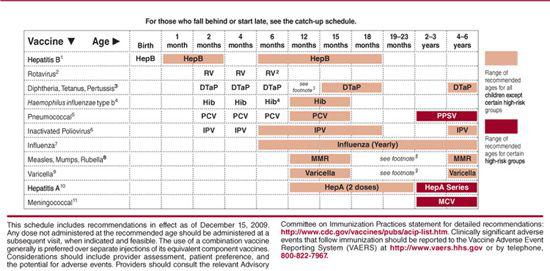

Table 244–2. Recommended Immunization Schedule for Persons Aged 7 Through 18 Years—United States, 2010
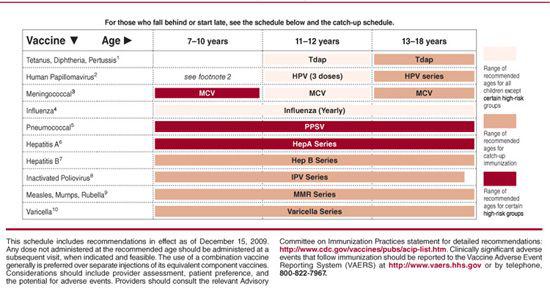

Table 244–3. Catch-up Immunization Schedule for Persons Aged 4 Months Through 18 Years Who Start Late or Who Are More Than 1 Month Behind—United States, 2010
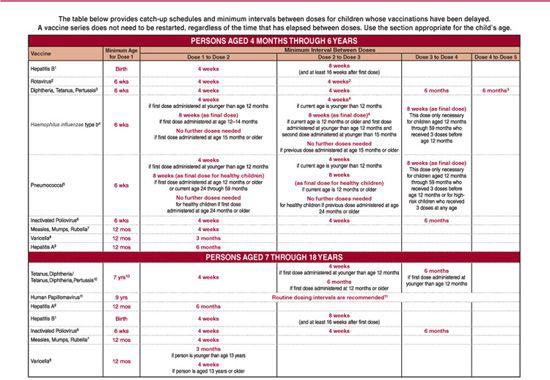

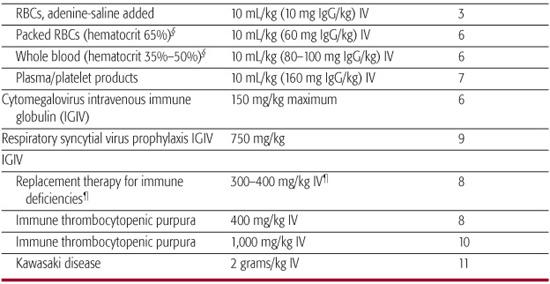
Table 244–4. Suggested Intervals between Administration of Antibody-Containing Products and Vaccination for Measles or Varicella*
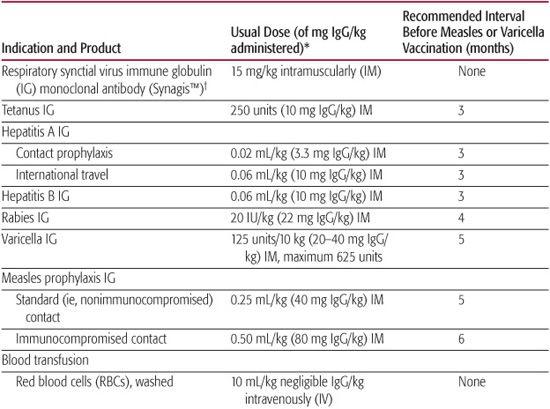

There are minimum ages for the administration of all routinely recommended vaccines except hepatitis B vaccine (Table 244-1). Likewise, there are minimum intervals between the doses of the same vaccine. Because vaccine administration before the recommended age or interval can result in decreased immunogenicity, the Advisory Committee on Immunization Practices recommends that doses administered more than 4 days early not be considered valid doses.
There are no minimum intervals between doses of the different inactivated vaccines, or between administration of live parenteral or oral vaccines and any other vaccine. However, live parenteral or intranasal vaccines not administered on the same day should be separated by at least 4 weeks. Likewise, there are no maximum intervals between doses of routinely recommended vaccines. When there is a lapse in the administration of sequential doses of vaccines in a given series, the series need not be restarted but may simply be continued. There is no harm in immunizing an individual who has already had the disease.
Antibody-containing products, including whole blood, packed red blood cells, plasma, hyperimmune globulin, and intravenous immune globulin, can interfere with the immune response to some live viral vaccines. Measles and varicella vaccine must be deferred for 3 or more months after the receipt of an antibody-containing product. The interval varies with the amount of antigen-specific antibody in the product received (Table 244-4).
When vaccines are administered according to the current schedule, a child may receive three or four injections in a single visit. The pain associated with the administration of immunizations may be a source of distress and anxiety for patients and their families.6 Parents can be coached to present a calm, matter-of-fact demeanor. They may be taught distraction techniques to minimize the discomfort experienced by their children. Sucrose water (12–50% or 1 packet of sugar in 10 mL of water) may decrease pain in children younger than 6 months when administered shortly before a procedure. When a child needs multiple injections at a single visit, simultaneous (multiple staff members injecting separate sites at the same time) is preferred to sequential (one after the other) administration. Topical anesthetics such as EMLA cream or vapocoolant sprays are not routinely recommended but may be necessary for selected children.
All vaccines undergo rigorous safety testing before licensure. However, no vaccine is completely risk-free. The value of a given vaccine depends on the prevalence and severity of the disease targeted, the vaccine’s ability to prevent or modify disease, and the incidence and severity of vaccine-related mortality. Although most adverse events after vaccination are minor and self-limited, some vaccines have been associated with rare but serious side effects.
In the United States, the National Vaccine Injury Compensation Program (VICP) was created by the National Childhood Vaccine Injury Act (NCVIA) of 1986. A no-fault alternative to civil litigation, the VICP was created to adjudicate vaccine injury claims and compensate individuals found to be injured by certain vaccines. Providers who administer covered vaccines in both the public and private sectors have specific responsibilities defined by the National Childhood Vaccine Injury Act. For example, patients and/or their parents or guardians must be informed of the risks and benefits of vaccines. A current version of the Vaccine Information Statement (VIS) for each covered vaccine must be provided each time the vaccine is administered (available at http://www.cdc.gov/nip/publications/VIS/default.htm).8 Providers must also document key components of the vaccination process, including the date of administration, the lot number, the manufacturer, the VIS version date, and the date the VIS was given. The National Childhood Vaccine Injury Act also requires health care providers to report adverse events following vaccination to the National Vaccine Adverse Event Reporting System (VAERS).9 VAERS reporting forms and more information are available at http://vaers.hhs.gov or by calling 800-882-7967.
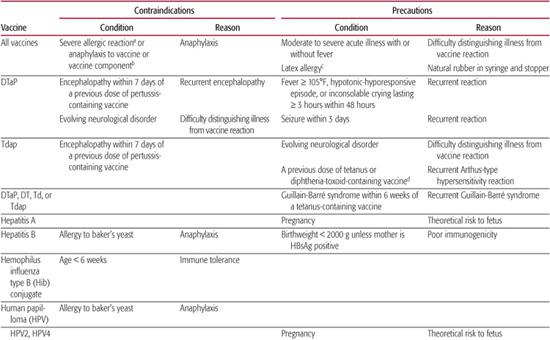
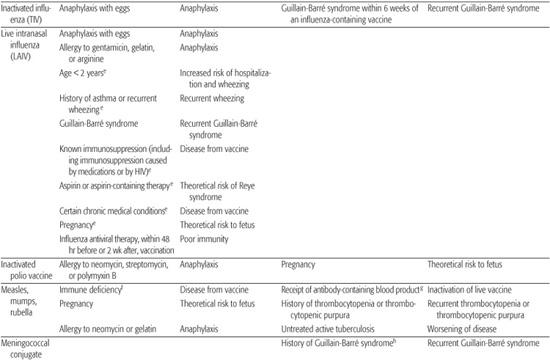
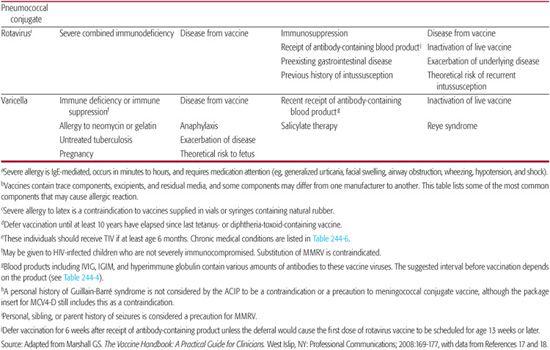
Table 244–5. Contraindications and Precautions to Further Vaccine Doses
Concerns about vaccine safety have eroded parental confidence in immunizations, leading to antivaccine movements in some countries and occasionally a resurgence of vaccine-preventable diseases.10,11 Increasingly, pediatric health care providers encounter families who refuse one or more immunization for their children. The American Academy of Pediatrics Committee on Bioethics has published guidance for pediatricians faced with vaccine refusal.12 Physicians should discuss the risks and benefits of vaccines, helping parents consider the risks of each individual vaccine with the risks of remaining unimmunized. Continued parental refusal of vaccines should be respected unless a child faces serious harm, as might be the case during an outbreak. However, communication about vaccines should continue at each subsequent health care visit. To facilitate the process of informed refusal, the AAP has developed a refusal waiver (http://cispimmunize.org/pro/pdf/RefusaltoVaccinate_revised%204-11-06.pdf) that outlines potential risks of vaccine refusal.13 It is suggested that this information be reviewed with the parent and a signature should be obtained at each medical encounter.
Thimerosal is a mercury-based compound used as a preservative in vaccines since the 1930s. Although the adverse health effects of other forms of organic mercury are well described, there is no scientific evidence of harm to infants from the low levels of ethyl mercury contained in thimerosal-containing vaccines. However, in 1999 the US Public Health Service and the American Academy of Pediatrics recommended that thimerosal be removed from vaccines as a precautionary measure.14 Thimerosal has since been eliminated as a preservative from all routinely recommended childhood vaccines except influenza vaccine; thimerosal-free influenza vaccine preparations are available. In a report issued in 2001, the Institute of Medicine found no evidence to prove a link between thimerosal-containing vaccines and autism, attention deficit hyperactivity disorder, or speech and language delay.15 Despite the removal of thimerosal from routine childhood vaccines, the incidence of autism continues to rise.16 This is significant evidence that thimerosal does not lead to autism.
The following sections provide information about each of the vaccines routinely recommended for children and adolescents. Other vaccines are available and recommended for specific populations based on increased susceptibility or potential for morbidity (eg, pneumococcal polysaccharide vaccine). Specific vaccine recommendations for travelers are discussed in Chapter 18. Additional detailed information about specific vaccines may be found in the American Academy of Pediatrics’ Red Book17 and the Centers for Disease Control and Prevention’s Pink Book.18
Table 244-5 contains information about contraindications and precautions for each vaccine.5,17-19 A contraindication is a condition in a potential vaccine recipient that increases the risk for a serious adverse reaction. When a contraindication is present, a vaccine should not be administered. A precaution is a condition in a potential vaccine recipient that may increase the risk for a serious adverse reaction, or that may compromise the ability of the vaccine to produce immunity. When a precaution is present, immunization is typically deferred, at least temporarily.
DIPHTHERIA–TETANUS–ACELLULAR PERTUSSIS VACCINE
Routine childhood immunization has virtually eliminated once-common diseases like diphtheria and tetanus and significantly reduced cases of pertussis. Diphtheria–tetanus–whole cell pertussis vaccines (DTwP) introduced in the 1940s were associated with high rates of local and systemic reactions. 
In contrast, diphtheria–tetanus–acellular pertussis (DTaP) vaccines contain diphtheria and tetanus toxoids combined with two or more other purified Bordetella pertussis antigens. Licensed DTaP vaccines and combination vaccines that include DTaP all contain pertussis toxin (PT), a cell wall protein that promotes attachment to respiratory epithelial cells, and filamentous hemaglutinin (FHA). Pertactin (PRN) and fimbrial proteins (FIM) are included in some DTaP vaccines. DTaP and DTaP-containing vaccine products and their pertussis antigen components are listed in eTable 244.1  . Combination vaccines include a DTaP–Hepatitis b–IPV vaccine (Pediarix [GlaxoSmithKline]), a DTaP-IPV/Hib vaccine (Pentacel [Sanofi Pasteur]), a DTaP-Hib vaccine (TriHIBit [Sanofi Pasteur]), and a DTaP-IPV vaccine (Kinrix [GlaxoSmithKline]). Pediarix is licensed for the infant series, whereas Pentacel is licensed for the infant and toddler doses. TriHIBit is licensed for only the fourth dose of the vaccine series, and Kinrix is licensed as the fifth DTaP and fourth IPV doses in children ages 4 to 6. A diphtheria and tetanus toxoids (DT) vaccine is available for children younger than age 7 who cannot receive pertussis vaccine.
. Combination vaccines include a DTaP–Hepatitis b–IPV vaccine (Pediarix [GlaxoSmithKline]), a DTaP-IPV/Hib vaccine (Pentacel [Sanofi Pasteur]), a DTaP-Hib vaccine (TriHIBit [Sanofi Pasteur]), and a DTaP-IPV vaccine (Kinrix [GlaxoSmithKline]). Pediarix is licensed for the infant series, whereas Pentacel is licensed for the infant and toddler doses. TriHIBit is licensed for only the fourth dose of the vaccine series, and Kinrix is licensed as the fifth DTaP and fourth IPV doses in children ages 4 to 6. A diphtheria and tetanus toxoids (DT) vaccine is available for children younger than age 7 who cannot receive pertussis vaccine.
More than 95% of infants develop protective antibody against diphtheria after four doses of vaccine, and clinical efficacy is estimated at 97%. Essentially 100% of infants develop protective antibody against tetanus. There are no serologic correlates of protection for pertussis, but point estimates of clinical efficacy range from 80% to 85%.
Children ages 6 months to 6 years should receive five doses of diphtheria–tetanus–acellular pertussis (DTaP) vaccine.  Adverse reactions—both mild and severe—are significantly less common with DTaP vaccines than with diphtheria–tetanus–whole cell pertussis (DTwP) vaccine. Mild reactions include injection site reactions such as redness, tenderness and swelling, fever > 101°F (2–7%), and irritability (12–30%). Swelling of an entire limb has been reported in children receiving four or five consecutive doses of DTaP vaccine. The swelling resolves without sequelae and does not preclude further doses of vaccine. Contraindications and precautions are listed in Table 244-5.
Adverse reactions—both mild and severe—are significantly less common with DTaP vaccines than with diphtheria–tetanus–whole cell pertussis (DTwP) vaccine. Mild reactions include injection site reactions such as redness, tenderness and swelling, fever > 101°F (2–7%), and irritability (12–30%). Swelling of an entire limb has been reported in children receiving four or five consecutive doses of DTaP vaccine. The swelling resolves without sequelae and does not preclude further doses of vaccine. Contraindications and precautions are listed in Table 244-5.
POLIOVIRUS VACCINE
With routine childhood immunization, the annual number of cases of paralytic polio in the United States fell from more than 40,000 in epidemic years to zero. The last indigenous case of paralytic polio in the United States occurred in 1979. Both live attenuated and inactivated poliovirus vaccines are used worldwide. An oral live attenuated poliovirus vaccine (OPV) was used routinely in the United States until 2000 but OPV rarely caused paralytic disease in vaccine recipients or their contacts,21 leading to the exclusive use of inactivated poliovirus vaccine in the United States since 2000.22
Inactivated poliovirus vaccine (IPV) contains formaldehyde-inactivated poliovirus types 1, 2, and 3 and 2-phenoxyethanol as a preservative. Trace amounts of streptomycin, neomycin, and polymyxin b may be present. The vaccine is immunogenic, with at least 90% of vaccines developing protective antibody after two doses and 99% after three doses. IPV is also available as part of combination vaccines.
All children should receive four doses of IPV. The final dose of IPV vaccine should be administered on or after the 4th birthday, and at least 6 months following the previous dose. If 4 doses are administered before the 4th birthday, a 5th dose should be administered at 4 to 6 years of age.  Like other injectable vaccines, IPV causes minor local reactions such as pain and redness at the insertion site. Allergic reactions attributed to trace amounts of streptomycin, neomycin, and polymyxin b in the vaccine have been reported. No serious adverse events have been attributed to IPV. Unlike the oral poliovirus vaccine (OPV) that was previously used in the United States, IPV cannot cause vaccine-associated paralytic polio because it does not contain any live virus.
Like other injectable vaccines, IPV causes minor local reactions such as pain and redness at the insertion site. Allergic reactions attributed to trace amounts of streptomycin, neomycin, and polymyxin b in the vaccine have been reported. No serious adverse events have been attributed to IPV. Unlike the oral poliovirus vaccine (OPV) that was previously used in the United States, IPV cannot cause vaccine-associated paralytic polio because it does not contain any live virus.
HEPATITIS B
More than 1 million people in the United States are chronically infected with hepatitis B vaccine. Before immunization of infants with hepatitis B vaccine became routine, up to 30% to 40% of chronic infections were thought to have resulted from perinatal or early-childhood transmission of the virus. Infants infected perinatally have a 90% risk of chronic infection, and up to 25% die of chronic liver disease as adults.23 Administration of hepatitis B vaccine at birth with or without hepatitis B immune globulin effectively reduces perinatal infection.24,25 Both universal immunization of infants at birth and effective postexposure prophylaxis of infants born to hepatitis B surface-antigen-positive mothers are key components of national immunization strategy to eliminate hepatitis B transmission.26 Two recombinant hepatitis B vaccines (Recombivax HB [Merck] and Energix B [Glaxo-SmithKline]) are licensed in the United States. Each is available in pediatric and adult formulations (eTable 244.2  ). Combination vaccines for the prevention of hepatitis B infection in children include a Haemophilus influenzae type b–hepatitis B vaccine (Comvax [Merck]) and a diphtheria–tetanus–acellular pertussis–hepatitis B–inactivated polio vaccine (Pediarix [GlaxoSmithKline]). A combination hepatitis A–hepatitis B vaccine (Twinrix [GlaxoSmithKline]) is licensed only for use in adults age 18 or older. Hepatitis B vaccines are efficacious, inducing a protective antibody response in over 99% of infant recipients after three doses.
). Combination vaccines for the prevention of hepatitis B infection in children include a Haemophilus influenzae type b–hepatitis B vaccine (Comvax [Merck]) and a diphtheria–tetanus–acellular pertussis–hepatitis B–inactivated polio vaccine (Pediarix [GlaxoSmithKline]). A combination hepatitis A–hepatitis B vaccine (Twinrix [GlaxoSmithKline]) is licensed only for use in adults age 18 or older. Hepatitis B vaccines are efficacious, inducing a protective antibody response in over 99% of infant recipients after three doses.
Three doses of hepatitis B vaccine are recommended for all infants. A dose of 0.5 mL is administered intramuscularly. Monovalent hepatitis B vaccine is indicated for all newborns before hospital discharge. Infants born to hepatitis B surface antigen (HBsAg)-positive mothers should receive the vaccine within 12 hours of birth along with 0.5 mL of hepatitis immune globulin (HBIG) administered at a separate site. When a mother’s HBsAg is unknown, infants should receive hepatitis B vaccine within 12 hours of birth and HBIG no later than age 1 week if the mother is subsequently found to be HBsAg-positive. Either monovalent vaccine or a combination hepatitis B vaccine may then be used to complete the series with subsequent doses given at age 1 to 2 months and 6 to 18 months. For infants born to HBsAg mothers, the third dose should be administered at 6 months of age.
Infants born to hepatitis B surface antigen (HBsAg)-positive mothers should be tested for HBsAg and antibody to HBsAg (anti-HBs) after completion of the vaccine series. Testing is generally performed at age 9 to 18 months or at the next well-child visit after completion of the primary series. HBsAg-negative infants with non-protective levels of anti-HBs (< 10 mIU/mL) should be reimmunized with three doses of hepatitis B vaccine at 2-month intervals. Serologic testing is not required for infants born to HBsAg-negative mothers.
Diminished immune responses have been noted in infants less than 2000 grams who receive hepatitis B vaccine before age 1 month. The immunization strategy for these infants depends on the hepatitis B surface antigen (HBsAg) status of the mother. When the maternal HBsAg status is positive or unknown, hepatitis B vaccine is administered within 12 hours of birth, but this dose is not counted in the three-dose series. Routine hepatitis B immunization should begin at age 1 month. For infants less than 2000 grams born to HBsAg-negative mothers, the first dose of hepatitis B vaccine is postponed until age 1 month or hospital discharge.
Adverse effects associated with hepatitis B vaccine include pain at the injection site in 3% to 9% of children vaccinated. Up to 20% of children experience mild systemic complaints including headache fatigue and irritability, and up to 6% experience low-grade fever. Serious adverse events are rare after hepatitis B immunization. Contraindications and precautions are listed in Table 244-5. 
HAEMOPHILUS INFLUENZA TYPE B (HIB) CONJUGATE VACCINE
Before licensure of conjugate vaccines for the prevention of Haemophilus influenzae type B (Hib) infection, one in 200 children developed invasive Hib disease (see Chapter 263).
Two single-antigen conjugate Hib vaccines are available in the United States. All vaccines contain Hib capsular polysaccharide (PRP) conjugated to either a tetanus toxoid (PRP-T, Act-Hib [Sanofi Pasteur]) or an outer-membrane protein from Neisseria meningitidis serogroup B (PRP-OMP, Pedvax HIB [Merck]). Hib-containing combination vaccinations include a Hib–hepatitis B vaccine (COMVAX [Merck]), a DTaP-IPV/Hib vaccine (Pentacel [Sanofi Pasteur]), and a DTaP/Hib vaccine licensed only for use at age 12 to 15 months (TriHIBit [Sanofi Pasteur]).
All Hib conjugate vaccines are immunogenic, inducing protective antibody against HIB polysaccharide in 95% of children after a primary series. Efficacy against invasive disease is estimated at 95% to 100%. However, PRPOMP is unique in its ability to elicit high levels of antibody in young children with the first dose of vaccine. Therefore, PRP-OMP-containing vaccines (Pedvax HIB [Merck]) are preferred for children at high risk of invasive Hib disease, including American Indian and Alaskan Native children.
The schedule for Hib vaccine differs by the product used and the age at initiation of the series.31 The primary series of PRP-T consists of three doses of vaccine given at ages 2 months, 4 months, and 6 months. The primary series of PRP-OMP consists of two doses given at ages 2 months and 4 months. The vaccines are considered interchangeable for the primary series, but when at least one dose of PRP-T is used, three doses are required. The first dose of the primary series should not be given to infants younger than age 6 weeks because of the risk of inducing immunologic tolerance against subsequent doses. A booster dose of Hib vaccine is given at age 12 to 15 months. When the first dose of Hib vaccine is not administered until age 7 to 11 months, a second dose is indicated 4 weeks after the first, with a booster at age 12 to 15 months. When the first dose of Hib vaccine is administered at age 15 months or later, subsequent doses are not needed.
Healthy children not immunized before age 5 do not require immunization with Hib vaccine. However, previously unvaccinated individuals older than age 59 months who are at increased risk for invasive Hib disease, including those with functional or anatomic asplenia, immunodeficiency, immunosuppression from chemotherapy, or HIV infection and recipients of a hematopoietic stem cell transplant, should be given at least one dose of a pediatric Hib vaccine.
Mild side effects from Hib vaccine occur in 25% of recipients and include low-grade fever, tenderness, redness, and swelling at the insertion site. Contraindications and precautions are listed in Table 244-5.
PNEUMOCOCCAL CONJUGATE VACCINE
S pneumoniae is a common cause of invasive and noninvasive bacterial infection in children worldwide. The organism colonizes the upper respiratory tract, and disease results from local spread (otitis media, sinusitis) or hematogenous dissemination (bacteremia, pneumonia, meningitis). Before the licensure of a 7-valent pneumococcal conjugate vaccine (PCV7 [Prevnar, Wyeth]) in 2000, pneumococci accounted for 17,000 cases of invasive disease each year in children under age 5, including 700 cases of pneumonia and 500 deaths. The highest rates of disease were seen in children ages 6 to 11 months and in Alaska Natives, African Americans, and specific American Indian populations. With universal immunization of young children, cases of invasive pneumococcal disease in children younger than age 5 decreased by 77%.32
PCV7 contained purified capsular polysaccharide from S pneumoniae serotypes 4, 6B, 9V, 14, 19F, and 18 C conjugated to CRM 197 (a nontoxic mutant of diphtheria toxin) and in pre-licensure trials involving ∼40,000 children, was 97% effective in reducing invasive pneumococcal disease due to vaccine serotypes.33 In 2010, a 13-valent pneumococcal conjugate containing the 7 original serotypes as well as serotypes 1, 3, 5, 6A, 7F, and 19A replaced PCV7 in the routine immunization schedule.
As with PCV7, a four-dose series of PCV13 is recommended at ages 2, 4, 6, and 12 to 15 months of age.33 The 0.5 mL dose is administered intramuscularly. Children previously immunized with one or more doses of PCV7 should complete the series with PCV13. When initiation of the series is delayed, the total number of recommended doses depends on the age at the first dose of vaccine. For example, when the first dose is given at 7 to 11 months of age, children should receive a 2-dose primary series (doses separated by at least 4 weeks) followed by a third dose at 12 to 15 months of age. A single dose of PCV13 is also recommended for children 15 to 59 months previously immunized with a complete series of PCV7. While routine PCV13 immunization is not recommended for children ≥ 5 years, this supplemental dose should be administered through age 71 months for previously immunized children with high-risk medical conditions. The minimum interval between doses of PCV7 and PCV13 is 8 weeks.
Adverse reactions following PCV13 include local reactions such as erythema and tenderness. Systemic reactions such as fever, decreased appetite, irritability, and increased or decreased sleep have also been reported.
MEASLES, MUMPS, RUBELLA (MMR) VACCINE
Since the licensure of an effective vaccine against measles, mumps, and rubella in the 1960s, the incidences of measles, mumps, rubella, and congenital rubella syndrome have decreased more than 99%. Sporadic outbreaks of disease occur in unvaccinated or incompletely vaccinated populations.34-36
Measles, mumps, rubella vaccine (MMR II [Merck]) contains three live attenuated viruses. Vaccine strains of measles and mumps are grown in chick embryo cell culture; rubella is grown in human diploid cell culture. A combination measles, mumps, rubella, varicella vaccine (MMRV, ProQuad [Merck]) is also available. Each vaccine contains small quantities of neomycin to prevent bacterial overgrowth.
A single dose of MMR vaccine confers protection to all three viruses in more than 95% of vaccine recipients vaccinated at age 12 months or older.37 Individuals who do not develop serologic evidence of immunity after the first dose of vaccine typically respond to a second dose. The duration of protective immunity is long lasting and probably lifelong.
Two doses of MMR vaccine are recommended for all children. A dose of 0.5 mL is administered subcutaneously. The first dose is administered at age 12 to 15 months, and the second dose at age 4 to 6 years. The second dose may be administered earlier as long as 4 weeks have elapsed since the first dose. During measles outbreaks or when the risk of exposure to measles is high, children as young as age 6 months may receive the vaccine, but doses administered before age 12 months are not counted in the two-dose series. 
MMR vaccine may be given simultaneously with any other live or inactivated childhood vaccines as long as the vaccines are given in separate syringes at separate sites. Injected live virus vaccines not given on the same day as MMR vaccine should be deferred for at least 4 weeks. Measles vaccination may temporarily suppress tuberculin skin test reactivity for 4 to 6 weeks after immunization but will not interfere with the accuracy of a test placed on the same day.
Redness, induration, or soreness at the injection site may occur after MMR vaccine. Local hypersensitivity consisting of wheal-and-flare reactions or urticaria is occasionally seen. Fever and rash are the most common systemic side effects, with 5% to 10% of vaccinees developing a fever ≥ 103°F 7 to 12 days after immunization and 5% developing a morbilliform rash. Higher rates of fever as well as febrile seizures have been reported with the combination MMRV vaccine. For children ages 12 to 23 months, the rate of febrile seizures after MMR vaccine is 4 cases per 10,000 vaccinations. In contrast, the rate of febrile seizure after MMRV vaccination is 9 per 10,000 vaccinations.38 Neither vaccine is associated with the development of chronic seizure disorders. Transient thrombocytopenia clustering 2 to 3 weeks after immunization has been observed in less than 1 in 30,000 vaccines and is presumably due to the measles component of the vaccine. The risk of thrombocytopenia with vaccination is less than that observed with natural measles infection. Arthralgias occur in up to 25% of adult women given rubella-containing vaccines, including MMR, but joint complaints in children are much less common.
In 1998, Dr. Andrew Wakefield and a group of investigators in Great Britain postulated a connection between receipt of MMR vaccine and autism.39 A number of well-controlled epidemiologic studies involving thousands of children, however, have failed to find evidence that MMR causes autism.40-42 Both the American Academy of Pediatrics and the Institute of Medicine have concluded that there is no association between MMR vaccine and autism.43,44
Contraindications and precautions to receiving MMR vaccine are listed in Table 244-5. Because of the risk of vaccine-induced thrombocytopenia, a history of immune thrombocytopenic purpura or thrombocytopenia is considered a precaution to MMR immunization. Although the measles and mumps viruses are grown in chick embryo cell culture, MMR does not contain significant quantities of cross-reacting egg proteins, and children with egg allergy may safely be vaccinated. Skin testing for egg allergy is not predictive of adverse reactions to vaccine and need not be performed.
Live virus vaccines including MMR are generally contraindicated in individuals known or suspected to be immunodeficient. However, HIV-infected children who are not severely immunocompromised (defined by age-specific quantitation of CD4 lymphocytes) should receive MMR because the risk associated with the vaccine is much less than associated with wild-type measles infection. MMRV vaccine should not be used in place of MMR vaccine for immunization of HIV-infected children. The combination MMRV vaccine should not be used in children younger than age 13. 
VARICELLA VACCINE
Before the licensure of varicella vaccine in 1995, approximately 4 million cases of chicken pox occurred annually in the United States.45 Although most infections were self-limited, secondary complications, including bacterial soft tissue infections, pneumonia, and encephalitis, resulted in more than 10,000 hospitalizations and 100 deaths each year.46,47 With routine childhood immunization, cases of varicella have decreased by 80%, while age-adjusted mortality from varicella has decreased by 66%.48,49
Two live attenuated varicella-zoster virus–containing vaccines are available for use in children. A monovalent varicella vaccine (Varivax [Merck]) is licensed for children older than age 12 months, adolescents, and adults. A combination measles, mumps, rubella, varicella vaccine (ProQuad [Merck]) is approved for use in children ages 12 months to 12 years. Both vaccines contain live attenuated varicella-zoster virus (Oka/Merck strain), although the combination vaccine contains a higher concentration of varicella vaccine virus, with a minimum of 10,000 plaque-forming units (PFU) compared to 1350 PFU in the monovalent product. Both vaccines contain minute quantities of neomycin and gelatin. 
A single dose of varicella vaccine is 87% effective against varicella disease.50,51 The efficacy of two doses of varicella vaccine administered at least 3 months apart is projected to be significantly higher (≥ 98%).
Because primary vaccine failure (failure to make protective antibodies) after one dose of varicella vaccine occurs and frequent school-based outbreaks of varicella have been observed in populations with high vaccination rates, two doses of varicella-containing vaccine are currently recommended for all children.53,54 The first dose is administered at age 12 to 15 months and the second dose is generally administered at age 4 to 6 years, although it can be given as early as 3 months after the first dose.55,56 The second dose may be administered as early as 3 months after the first.
Varicella vaccine is associated with few side effects. Approximately 20% of monovalent varicella vaccine recipients experience transient pain and tenderness at the site of injection. A mild varicelliform eruption develops at the injection site in 3% of children within 1 month of immunization, and 5% of children may develop a generalized rash. Transmission of attenuated vaccine virus from immuno-competent individuals with a rash after vaccination to susceptible individuals is extremely rare (1 case of transmission per 10 million doses).
Precautions and contraindications to varicella vaccine are listed in Table 244-5. Monovalent varicella vaccine does not contain egg protein and thus may be safely administered to individuals with egg allergy. Although the measles and mumps vaccine viruses in MMRV are grown in chicken-embryo cell culture, the amount of egg cross-reacting proteins is not significant, and children with egg allergy may also receive MMRV.
Specific recommendations have been published for immunization of individuals with altered immunity.17 Varicella-containing vaccines are not recommended for children with leukemia, lymphoma, or other malignancies affecting the bone marrow or lymphatic systems; children receiving long-term immunosuppressive therapy; or children with most congenital or acquired T-cell immunodeficiencies. Immunodeficiency should be excluded in children with a family history of hereditary immunodeficiency before a varicella-containing vaccine is administered. Immunization should be deferred in individuals receiving high-dose systemic corticosteroids for ≥ 14 days (≥ 2 mg/kg day of prednisone or its equivalent or 20 mg/day of prednisone), with a minimum interval of 1 month between the last dose of steroids and subsequent vaccination. Monovalent varicella vaccine may be administered to children with humoral immune deficiencies, and HIV-infected children in CDC Class 1 with a CD4 percentage of ≥ 15%. Because MMRV contains a higher concentration of varicella vaccine virus, it should not be administered to HIV-infected individuals.
Varicella vaccine is contraindicated in pregnant women, but children living in the same household may be vaccinated. Caution is advised when vaccinating children taking salicylates. No adverse events associated with salicylate use and varicella vaccine have been reported. However, the manufacturer recommended that salicylates be avoided for 6 weeks after vaccine administration because of the well-established relationship between Reye syndrome and use of salicylates during natural varicella infection. According to the Advisory Committee on Immunization Practices, varicella vaccine with subsequent close monitoring should be considered for children requiring chronic salicylate therapy because the risk of aspirin-associated complications is likely to be greater with natural varicella infection.
HEPATITIS A VACCINE
Before the implementation of a targeted hepatitis A immunization program in the United States, there were 180,000 infections annually, resulting in approximately 100 deaths (see Chapter 308).
Two hepatitis A vaccines are currently licensed for use in children age 12 months or older (Havrix [GlaxoSmithKline] and VAQTA [Merck]). Virtually 100% of children develop protective antibody after two doses of either hepatitis A vaccine. A combination hepatitis A–hepatitis B vaccine (Twinrix [GlaxoSmithKline]) is licensed only for use in adults age 18 or older.
Beginning in 1996, routine hepatitis A immunization was recommended only for children age 2 or older living in communities with high rates of hepatitis A infection or periodic outbreaks, namely children living in Alaska Native villages or Native Americans.
In 2005, hepatitis A vaccine was licensed for children as young as age 12 months, and the CDC amended recommendations to include all children beginning at age 1, regardless of state of residence.57 Two doses of hepatitis A vaccine are currently recommended for all children in the second year of life, with at least 6 months between doses. If immunity against hepatitis A is desired, any unimmunized child 2 to 18 years may be vaccinated. Additional recommendations are given in Table 244-1.58
Local injection site reactions, including erythema, swelling, and tenderness, occur in approximately 20% of children immunized with hepatitis A vaccine. Contraindications and precautions are listed in Table 244-5.
INFLUENZA VACCINE
Annual epidemics of influenza result in 50 to 60 million infections, with the highest attack rates in school-age children.59 The risk for serious disease, hospitalization, and death from influenza are highest among children age 2 and younger, adults age 65 and older, and individuals of any age with underlying medical conditions that place them at increased risk for complications from influenza.
Two different types of influenza vaccines are available for use in children. The trivalent inactivated vaccine (TIV) contains two inactivated influenza A viruses (types H1N1 and H3N2) and one influenza B virus. The composition of the vaccine changes annually based on circulating influenza types. Vaccine viruses are grown in chicken eggs and then subsequently inactivated. The dose varies by age but is always given intramuscularly (eTable 244.3  ). In general, TIV is approved for use in individuals age 6 months or older, including those with underlying medical conditions. Unlike other vaccines routinely recommended for children, some influenza vaccines contain small amounts of the preservative thimerosal.
). In general, TIV is approved for use in individuals age 6 months or older, including those with underlying medical conditions. Unlike other vaccines routinely recommended for children, some influenza vaccines contain small amounts of the preservative thimerosal.
A live attenuated influenza vaccine (LAIV) also contains two influenza A viruses and one influenza B virus. The viruses are reassortants that contain genes encoding for the surface proteins hemagglutinin and neuraminidase from wild-type influenza viruses and genes from master influenza A and B viruses that confer cold adaption and temperature sensitivity. Consequently, the vaccine viruses replicate in the cooler temperatures of the nasopharynx and are thus able to induce immunity, but they cannot replicate in the lower airways and cause disease. LAIV is licensed for use in healthy individuals ages 2 to 49.
The efficacy of influenza vaccines varies by vaccine type, the number of doses administered, the age of the vaccinee, and the match between circulating strains of influenza and those contained in the vaccine. In one study of children ages 1 to 15, TIV was 77% to 91% effective against culture-confirmed influenza A infection.60 Other studies have suggested lower efficacy, particularly against influenza B. No effectiveness is observed when previously unvaccinated children younger than age 9 receive a single dose of vaccine.61,62
In contrast to trivalent inactivated vaccine (TIV), live attenuated, cold-adapted influenza vaccine (LAIV) confers protection against influenza infection, even during seasons when there is a poor match between circulating strains of influenza and the vaccine strains. In a randomized, double-blind, placebo-controlled trial of children ages 15 to 71 months during a season in which circulating and vaccine strains were well matched, the efficacy of LAIV against culture-confirmed influenza was 89% in children who received one dose of vaccine and 94% in children who received two doses of vaccine. During the second season of the study, the circulating influenza A (H3N2) was not well matched to the vaccine strain, but the efficacy of LAIV was still 86%.63,64 Several comparative trials have demonstrated superior efficacy of LAIV over TIV in children.65,66
Annual influenza immunization is recommended for all children ages 6 months to 18 years. No influenza vaccine is licensed for use in children younger than age 6 months.67 Therefore, immunization of household contacts is the preferred way to protect these children from influenza.  Higher risk groups targeted for administration of the influenza vaccine are given in Table 244-6.
Higher risk groups targeted for administration of the influenza vaccine are given in Table 244-6.
Trivalent inactivated vaccine is ideally administered during October or November, whereas live attenuated, cold-adapted influenza vaccine can be administered as soon as the vaccine is available in late summer or early fall.
Transient, local reactions, including tenderness, erythema, and induration at the injection site, occur in 15% to 20% of trivalent inactivated vaccine recipients. Systemic reactions like fever and myalgias occur in 1% or less. Additional contraindications and precautions are listed in Table 244-5.
Nasal congestion is the most common adverse event observed after live attenuated, cold-adapted influenza vaccine, occurring in up to 59% of vaccinees younger than age 6. Decreased appetite and irritability occur less commonly after vaccination, whereas up to approximately 10% of vaccinees experience fever of 100°F to 102°F.
Live attenuated, cold-adapted influenza vaccine (LAIV) administration is associated with an increased risk of wheezing and hospitalization in children younger than age 2 and is not licensed for use in that population, nor in individuals with a history of asthma, reactive airway disease, or chronic lung disease. LAIV is not indicated for individuals with other chronic medical conditions, those who require chronic aspirin therapy, or individuals older than age 50. Like trivalent inactivated vaccine, the vaccine is produced in eggs and is contra-indicated in individuals with a history of severe egg allergy. Additional contraindications and precautions are listed in Table 244-5.
ROTAVIRUS VACCINE
Rotavirus is the most common cause of severe gastroenteritis in infants and children worldwide; nearly all children experience infection by age 5. In the United States, rotavirus gastroenteritis results in 55,000 to 70,000 hospitalizations annually and 20 to 60 deaths (see Chapter 236). 
Table 244–6. Target Groups for Influenza Immunization



