Immunizations
Alexander G. Fiks
IMMUNIZATION SCHEDULE
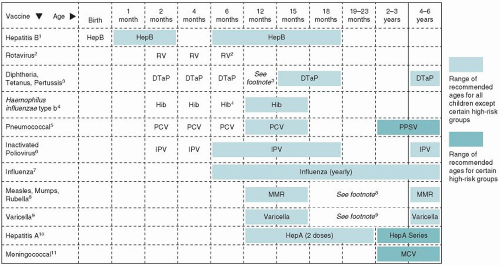
Figure 5-1 presents the recommended childhood immunization schedule for children and adolescents. The following are important general issues to consider:
The simultaneous administration of multiple vaccines that are routinely recommended for infants, children, and adolescents is preferred. When available, combination vaccines are generally preferable to separate injections.
A lapse in the immunization schedule does not require restarting a vaccine series. If a dose of vaccine is missed, it may be given at the next visit as long as it is still indicated. Many vaccines have upper age limits.
Increasing numbers of vaccinations are required for teens. It is important to review the vaccine history at each adolescent visit.
Immunizations, especially live virus vaccines, may be contraindicated in children with acquired or congenital immune deficiencies.
Due to shortages and changing recommendations, the immunization schedule is continuously being updated. Be sure to consult the current schedule when making vaccine decisions.
ADMINISTRATION GUIDELINES
Procedures
Federal law requires providers to distribute information on vaccines, available from the Centers for Disease Control and Prevention (CDC), before each vaccination (www.cdc.gov/vaccines/pubs/vis/default.htm).
Sites
The preferred sites for the administration of vaccines are the anterolateral aspect of the upper thigh (subcutaneous or intramuscular), the deltoid area of the upper arm (intramuscular), or the upper, outer triceps area of the upper arm (subcutaneous). In children >1 year, intramuscular injections should be administered in the anterolateral aspect of the thigh. For children >1 year, the deltoid muscle is the preferred site of administration. Generally, the upper outer aspect of the buttocks should not be used for immunizing an infant.
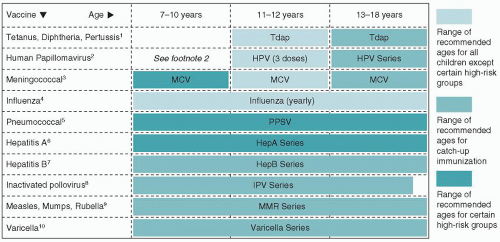 FIGURE 5-1 Recommended childhood immunization schedule United States, 2010. Immunization schedules courtesy of the Centers for Disease Control and Prevention, Atlanta, GA, 2010. |
Diphtheria, Tetanus, and Acellular Pertussis (DTaP) Vaccine
IM: 0.5 mL. The Advisory Committee on Immunization Practices and the American Academy of Pediatrics (AAP) recommend four doses for primary immunization, with the first three administered at 4- to 8-week intervals starting at 2 months of age, and the fourth dose 6 months after the third dose and after 1 year of age. A fifth dose is recommended before school entry, at the age of 4 to 6 years. Subsequent boosters should be administered starting at 11 years of age using combined tetanus, diphtheria, and pertussis (Tdap) vaccine. Tdap should only be given once. Tetanus-diphtheria (Td) vaccine should be used for all subsequent boosters and given at 10-year intervals throughout life. An incomplete DTaP series is a common reason for vaccine delay in infants and toddlers.
Tetanus Immune Globulin
IM: 250 U (intramuscular) as a single dose for postexposure prophylaxis. The need for prophylaxis depends on wound cleanliness and severity, as well as prior tetanus toxoid (TT) vaccination history. TT is given along with immune globulin.
Contraindications
While adverse reactions are far less common than prior to the development of the acellular pertussis vaccine, contraindications for DTaP include the following:
An immediate anaphylactic reaction
Encephalopathy within 7 days
A progressive neurological disorder (infantile spasms) until the condition has stabilized and the diagnosis clarified
Haemophilus influenza Type b Conjugate (Hib) Vaccine
IM: Three or four 0.5-mL doses are administered at 2 months, 4 months, 6 months, and 12 to 15 months of age. If a three-dose product is used, the third dose may be omitted. In situations in which the brand of vaccine used previously is not known, four doses are required. Hib vaccine is not required after 60 months of age.
Hepatitis A Vaccine (HAV)
IM: HAV 25U (VAQTA) or 720 EL.U (HAVRIX) in the deltoid muscle is recommended for the routine vaccine of all children beginning at 12 months of age. The second dose should be given 6 months later. For children and adolescents >23 months of age, HAV is recommended in areas where programs target older children, for those at increased risk, and for those desiring immunity.
Hepatitis B Vaccine (HBV)
IM: The first dose is ideally administered at birth in the neonatal nursery and must then be added to a child’s primary care vaccination record. The next dose is administered 1 to 4 months later, most commonly at 1 to 2 months of age. The third dose is recommended beginning at 6 months of age and must be given 8 weeks after the second dose and 16 weeks after the first dose. It is acceptable to administer a fourth dose of HBV if combination products are used.
Hepatitis B Immune Globulin (HBIG)
Infants born to HBsAg-positive mothers: Both HBV (0.5 mL) and HBIG are recommended within 12 hours after birth. The second dose of HBV is then administered at 1 to 2 months of age and the third dose at 6 months of age. Hepatitis B surface antigen and hepatitis B surface antibody should then be checked starting at 9 months of age or the first preventive visit after completion of the series.
Infants born to mothers with HBsAg unknown: Mothers should be tested on admission for delivery. Administer HBV by 12 hours of age. HBIG should be administered as soon as possible and within 7 days of birth if the mother tests HBsAg positive.
Human Papilloma Virus (HPV) Vaccine
IM: 0.5-mL doses should be administered beginning at 11 to 12 years of age. The second dose is given 1 (HPV2) or 2 months (HPV4) after the first dose, and the third dose is administered 6 months after the first. Women may receive the vaccine up to 26 years of age if they have not completed the full series and are not pregnant. The vaccine may also be offered to males in this age group to help prevent genital warts. Of all adolescent vaccines, rates of completion are currently lowest for the HPV series.
Measles-Mumps-Rubella (MMR) Vaccine
SC: 0.5 mL at age 12 to 15 months. A booster dose is recommended at 4 to 6 years of age.
Contraindications
Contraindications to measles, mumps, and rubella vaccination include serious illness (not simple fever or upper respiratory infections), pregnancy, anaphylactic reaction to gelatin or neomycin, low platelets, recent injection of immune globulin, high-dose steroid use for at least 14 days, and compromised immunity (see discussion of HIV later). Autism is not associated with receipt of the MMR vaccine.
 HINT: In patients who have been exposed to the measles virus, the vaccine may be given within 72 hours of exposure to provide protection. Alternatively, if given within 6 days of exposure, immune globulin (0.25 mL/kg, IM) may prevent or modify measles in a susceptible person. Children and adolescents with symptomatic HIV infections who are exposed to measles should receive immune globulin prophylaxis (0.5 mL/kg) regardless of their immunization status. The maximum dosage for all patients is 15 mL.
HINT: In patients who have been exposed to the measles virus, the vaccine may be given within 72 hours of exposure to provide protection. Alternatively, if given within 6 days of exposure, immune globulin (0.25 mL/kg, IM) may prevent or modify measles in a susceptible person. Children and adolescents with symptomatic HIV infections who are exposed to measles should receive immune globulin prophylaxis (0.5 mL/kg) regardless of their immunization status. The maximum dosage for all patients is 15 mL.Meningococcal Conjugate Vaccine (MCV4)
IM: Meningococcal vaccine is recommended beginning at 11 to 12 years of age for all teens. One 0.5-mL dose is currently required. The vaccine should be avoided in those with a history of Guillain-Barré syndrome. MCV4 is now recommended instead of meningococcal polysaccharide vaccine (MPS4) for children between 2 and 10 years of age at increased risk of meningococcal disease. In this age group,
the primary recipients are children with terminal complement component deficiencies, those who have anatomic or functional asplenia, and those traveling to areas with especially high rates of disease. Based on developments at the time of publication, a booster dose for MCV4 is likely to be needed at age 16 years or 5 years after the first dose. Consult the current vaccine schedule.
the primary recipients are children with terminal complement component deficiencies, those who have anatomic or functional asplenia, and those traveling to areas with especially high rates of disease. Based on developments at the time of publication, a booster dose for MCV4 is likely to be needed at age 16 years or 5 years after the first dose. Consult the current vaccine schedule.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree