Because the field of immunizations is rapidly changing, it is important for healthcare providers to seek the most cup-to-date information available. The immunization recommendations outlined in this chapter are current but will change as technology evolves and our understanding of the epidemiology of vaccine-preventable diseases changes. The most useful sources for regularly updated information about immunization are the following:
1. National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention (CDC). Maintains a website with extensive vaccine-related resources, including the recommendations of the Advisory Committee on Immunization Practices (ACIP), vaccination schedules, Vaccine Information Statements, information for the public and providers, and links to other vaccine materials. Available at: http://www.cdc.gov/vaccines.
2. CDC Contact Center. The CDC-INFO contact center provides services to the public and healthcare professionals regarding a variety of health-related issues, including immunizations; available 24 hours a day, 7 days a week, at 1-800-232-4636 (English and Spanish).
3. The Red Book: Report of the Committee on Infectious Diseases. Published at 2- to 3-year intervals by the American Academy of Pediatrics (AAP). The 2012 Red Book is available from the AAP. Updates are published in the journal Pediatrics and can also be accessed at http://aapredbook.aappublications.org.
4. Immunization Action Coalition. This nonprofit organization creates and distributes educational materials for healthcare providers and the public related to vaccines. All materials are provided free of charge and can be accessed at http://www.immunize.org.
5. Morbidity and Mortality Weekly Report (MMWR). Published weekly by the CDC. Available at: http://www.cdc.gov/mmwr.
STANDARDS FOR PEDIATRIC IMMUNIZATION PRACTICES
In the United States, every infant requires more than 25 doses of vaccine by age 18 months to be protected against 14 or more childhood diseases. In 2011, immunization coverage rates for children aged 19–35 months were more than 90% for poliovirus, measles-mumps-rubella, varicella, and hepatitis B vaccines, and were steadily increasing for more recently recommended vaccines such as pneumococcal conjugate, rotavirus, and hepatitis A vaccines. However, achieving and maintaining high immunization coverage rates remains challenging. The CDC recommends the following specific proven strategies to increase vaccination coverage rates: (1) assessing and providing feedback on practice/provider immunization rates; (2) keeping accurate immunization records; (3) recommending vaccination to parents, and reinforcing when to return for vaccination; (4) sending reminder messages to parents; (5) sending reminder messages to providers; (6) reducing missed opportunities to vaccinate; and (7) reducing barriers to immunize within the practice.
The National Childhood Vaccine Injury Act of 1986 requires that for each vaccine covered under the Vaccine Injury Compensation Program, caretakers should be advised about the risks and benefits of vaccination in a standard manner, using Vaccine Information Statements (VIS) produced by the CDC. Each time a Vaccine Injury Compensation Program–covered vaccine is administered, the current version of the VIS must be provided to the nonminor patient or legal caretaker. Vaccination documentation that is required in the medical record includes the vaccine manufacturer, lot number, and date of administration and expiration. The VIS version and date, and site and route of administration should also be recorded.
Needles used for vaccination should be sterile and disposable to minimize the opportunity for contamination. A 70% solution of alcohol is appropriate for disinfection of the stopper of the vaccine container and of the skin at the injection site. A 5% topical emulsion of lidocaine-prilocaine applied to the site of vaccination for 30–60 minutes prior to the injection minimizes the pain, especially when multiple vaccines are administered.
Compliance with the manufacturer’s recommendations for route and site of administration of injectable vaccines are critical for safety and efficacy. With few exceptions (intradermal influenza vaccine and Bacillus Calmette-Guérin [BCG] vaccine), all vaccines are given either intramuscularly or subcutaneously. All vaccines containing an adjuvant must be administered intramuscularly to avoid granuloma formation or necrosis. Intramuscular injections are given at a 90-degree angle to the skin, using a needle that is sufficiently long to reach the muscle tissue, but not so long as to injure underlying nerves, blood vessels, or bones. The anterolateral thigh is the preferred site of vaccination in newborns and children up to 2 years of age, and the deltoid muscle of the arm is the preferred site for children aged 3–18 years. Needle length and location should be as follows: ⅝ inch in newborn infants in the thigh; 1 inch in infants 1- to 12-month-olds (thigh), 1–1¼ inches in 1- to 18-year-olds (thigh), and ⅝–1 inch in 1- to 18-year-olds (deltoid). Subcutaneous injections should be administered at a 45-degree angle into the anterolateral aspect of the thigh (for infants younger than 12 months) or the upper outer triceps area (for children 12 months and older) using a 23- or 25-gauge, ⅝-inch needle. Pulling back on the syringe prior to vaccine injection (aspiration) is not required in CDC recommendations. A separate syringe and needle should be used for each vaccine.
Many combinations of vaccines can be administered simultaneously without increasing the risk of adverse effects or compromising immune response. Inactivated vaccines can be given simultaneously with, or at any time after, a different vaccine. Injectable or intranasal live-virus vaccines, if not administered on the same day, should be given at least 4 weeks apart (eg, measles-mumps-rubella [MMR] and varicella [VAR]). Extra doses of hepatitis B (HepB), Hib, MMR, and VAR are not harmful, but repetitive exposure to tetanus vaccine beyond the recommended intervals can result in hypersensitivity reactions and should be avoided. If an immunoglobulin (Ig) or blood product has been administered, live-virus vaccination should be delayed 3 to 11 months, depending on the product, to avoid interference with the immune response (eg, 3 months for tetanus Ig, hepatitis B Ig, and pooled Ig for hepatitis A; 5–6 months for measles Ig or cytomegalovirus Ig; and 11 months for intravenous Ig for Kawasaki disease).
With the large number of vaccine preparations available, interchangeability of vaccines is an issue. All brands of Hib conjugate, HepB, and hepatitis A (HepA) vaccines are interchangeable. For vaccines containing acellular pertussis antigens, it is recommended that the same brand be used, but when the brand is unknown or the same brand is unavailable, any vaccine with diphtheria and tetanus toxoids and acellular pertussis should be used to continue vaccination. Longer than recommended intervals between vaccinations does not reduce final antibody titers, and lapsed schedules do not require restarting the series.
The numerous vaccines and other immunologic products used in routine practice vary in the storage temperatures required. The majority of vaccines should never be subjected to freezing temperatures. Varicella-containing vaccines (MMRV, VAR, and herpes zoster) should be stored frozen. Product package inserts should be consulted for detailed information on vaccine storage conditions and shelf life.
Vaccines very rarely cause acute anaphylactic-type reactions. All vaccine providers should have the equipment, medications, staff, established protocols, and training to manage emergencies that may occur following vaccination.
CDC: General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(RR-02):1 [PMID: 21293327].
CDC: National and state vaccination coverage among children aged 19–35 months—United States, 2011. MMWR 2012;61:689 [PMID: 22951450].
ROUTINE CHILDHOOD & ADOLESCENT IMMUNIZATION SCHEDULES
Each year, the CDC recommends immunization schedules for children and adolescents. While variation from these schedules may be necessitated by epidemiologic or individual clinical circumstances, these schedules are an important guide for vaccination providers. Vaccines in the schedules are roughly ordered by the age at which the vaccines are first given. For example, HepB vaccination begins at birth, followed at 2 months of age by rotavirus, diphtheria-tetanus-acellular pertussis (DTaP), Hib, pneumococcal conjugate 13-valent (PCV13), and inactivated poliovirus (IPV) vaccines. Table 10–2 sets forth the 2014 schedule of routine immunizations for normal infants, children, and adolescents from birth through 18 years of age. Table 10–3 presents the 2014 schedule for persons aged 4 months through 18 years who started vaccination late or are more than 1 month behind the routine immunization schedule. Updated immunization schedules are available at http://www.cdc.gov/vaccines.
Combination vaccines help solve the problem of large numbers of injections during a clinic visit. Currently available combination vaccines include MMR, measles-mumps-rubella-varicella (MMRV), and various combinations of Hib, HepB, IPV, and DTaP, including DTaP-HepB-IPV and DTaP-IPV-Hib combination vaccines. Separate vaccines should not be combined into one syringe by the provider unless approved by the Food and Drug Administration (FDA), because this could decrease the efficacy of components in the vaccine.
Table 10–2. Recommended immunization schedule for persons aged 0 through 18 years – United States, 2014. (For those who fall behind or start late, see the catch-up schedule [Table 10–3]).

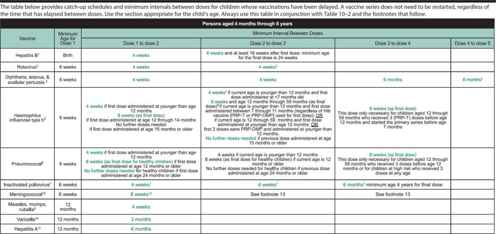
Table 10–3. Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind —United States, 2014.


VACCINE SAFETY
 Vaccine Safety Monitoring
Vaccine Safety Monitoring
The United States has a sophisticated, multifaceted system to monitor the safety of licensed vaccines. The Vaccine Adverse Events Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network each provide distinct contributions to the ongoing monitoring of vaccine safety. VAERS is a national passive surveillance system administered jointly by the FDA and CDC. VAERS accepts reports from healthcare providers and the public about possible vaccine-related adverse events. Reports of adverse events possibly related to vaccination may be made via the Internet (http://vaers.hhs.gov) or by telephone (1-800-822-7967). As a passive surveillance system, VAERS is subject to certain limitations, including underreporting, overreporting, the reporting of events that are temporally but not causally related to vaccination, and the lack of denominator data. The VSD, in comparison, is an active surveillance system with continuous safety monitoring of vaccines in defined patient populations. The VSD can conduct timely investigations of newly licensed vaccines or emerging vaccine safety concerns. The CISA Network is designed to develop protocols for the evaluation, diagnosis, and treatment of adverse events following immunization, and to provide a better understanding of adverse events following immunization at the individual level. Patients with rare and serious adverse events following immunization can be referred to the CISA Network for evaluation.
 Vaccine Risk-Benefit Communication
Vaccine Risk-Benefit Communication
Most parents in the United States choose to vaccinate their children: in 2011, less than 1% of young children had received no vaccines. However, parents’ concerns about vaccines are on the rise, and an increasing number of parents are choosing to delay or decline vaccination for their children. While there are myriad reasons why some parents may not vaccinate, several themes recur. Some parents do not believe their children are at risk for diseases such as poliomyelitis, measles, and tetanus. Other parents do not believe that certain vaccine-preventable diseases, such as varicella and pertussis, are particularly serious. There are also widespread concerns about the safety of vaccines. In a recent survey of more than 1500 parents, one-quarter believed that vaccines can cause autism in healthy children (despite no scientific evidence to support this claim), and more than one in 10 parents had refused at least one recommended vaccine. Healthcare providers have a critically important role in discussing the known risks and benefits of vaccination with parents. In this context, it is important that providers recognize that parent decisions are often based on inaccurate information about vaccine risk provided by the media or Internet sources. Parents with questions about vaccine safety should be directed to trusted websites, such as those of the AAP, the American Academy of Family Physicians (AAFP), the CDC (http://www.cdc.gov/vaccines), and the Immunization Action Coalition (http://www.immunize.org).
 Vaccine Contraindications and Precautions
Vaccine Contraindications and Precautions
All vaccines have certain contraindications and precautions that guide their administration. A contraindication indicates that the potential vaccine recipient is at increased risk of a serious adverse event. A vaccine should not be given when a contraindication to that vaccine is present, whereas a precaution indicates a circumstance that might increase the risk of adverse events or diminish the effectiveness of the vaccine. In the setting of precautions, the risks and benefits of vaccination must be carefully weighed prior to a decision regarding vaccination. Precautions are often temporary, in which case vaccination can resume once the precaution no longer applies. Contraindications and precautions are listed below with each vaccine. Additional, more detailed, information is available from the CDC (http://www.cdc.gov/vaccines), in the AAP’s Red Book, and in vaccine package inserts.
Baggs J et al: The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127 (Suppl 1):S45 [PMID: 21502240].
Freed GL et al: Parental vaccine safety concerns in 2009. Pediatrics 2010;125:654 [PMID: 20194286].
Glanz JM et al: A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr 2013;167:274 [PMID: 23338829].
VACCINATION IN SPECIAL CIRCUMSTANCES
 Minor Acute Illnesses
Minor Acute Illnesses
Minor acute illnesses, with or without low-grade fever, are not contraindications to vaccination, because there is no evidence that vaccination under these conditions increases the rate of adverse effects or decreases efficacy. A moderate to severe febrile illness may be a reason to postpone vaccination. Routine physical examination and temperature assessment are not necessary before vaccinating healthy infants and children.
 Children with Chronic Illnesses
Children with Chronic Illnesses
Most chronic diseases are not contraindications to vaccination; in fact, children with chronic diseases may be at greater risk of complications from vaccine-preventable diseases, such as influenza and pneumococcal infections. Premature infants are a good example. They should be immunized according to their chronologic, not gestational, age. Vaccine doses should not be reduced for preterm or low-birth-weight infants. One exception to this rule is children with progressive central nervous system disorders. Vaccination with DTaP should be deferred until the child’s neurologic status has been clarified and is stable.
 Immunodeficient Children
Immunodeficient Children
Congenitally immunodeficient children should not be immunized with live-virus vaccines (oral polio vaccine [OPV, available only in developing countries], rotavirus, MMR, VAR, MMRV, yellow fever, or live-attenuated influenza vaccine [LAIV]) or live-bacteria vaccines (BCG or live typhoid fever vaccine). Depending on the nature of the immunodeficiency, other vaccines are safe, but may fail to evoke an immune response. Children with cancer and children receiving high-dose corticosteroids or other immunosuppressive agents should not be vaccinated with live-virus or live-bacteria vaccines. This contraindication does not apply if the malignancy is in remission and chemotherapy has not been administered for at least 90 days. Live-virus vaccines may also be administered to previously healthy children receiving low to moderate doses of corticosteroids (defined as up to 2 mg/kg/d of prednisone or prednisone equivalent, with a 20 mg/d maximum) for less than 14 days; children receiving short-acting alternate-day corticosteroids; children being maintained on physiologic corticosteroid therapy without other immunodeficiency; and children receiving only topical, inhaled, or intra-articular corticosteroids.
Contraindication of live-pathogen vaccines also applies to children with HIV infection who are severely immunosuppressed. In general, those who receive MMR should have at least 15% CD4 cells, a CD4 lymphocyte count equivalent to CDC immunologic class 2, and be asymptomatic from their HIV. MMR for these children is recommended at 12 months of age (after 6 months during an epidemic). For HIV-infected children, a booster MMR dose may be given at least 1 month after the initial dose; in fact, giving this booster dose earlier than at 4–6 years of age is often encouraged. The booster dose may be given as early as 1 month later, but doses given before 1 year of age should not be considered part of a complete series. VAR vaccination is also recommended for HIV-infected children with CD4 cells preserved as listed above. The ACIP recommends only IPV vaccination for all children. Thus, immunodeficient children should no longer be exposed to OPV through household contacts. MMR and VAR are not contraindicated in household contacts of immunocompromised children. The recommended immunization schedule for immunocompromised children is available at http://www.cdc.gov/vaccines/pubs/pinkbook/.
 Allergic or Hypersensitive Children
Allergic or Hypersensitive Children
Hypersensitivity reactions are rare following vaccination (1.53 cases per 1 million doses). They are generally attributable to a trace component of the vaccine other than to the antigen itself; for example, MMR, IPV, and VAR contain microgram quantities of neomycin, and IPV also contains trace amounts of streptomycin and polymyxin B. Children with known anaphylactic responses to these antibiotics should not be given these vaccines. Trace quantities of egg antigens may be present in both inactivated and live influenza and yellow fever vaccines. Guidelines for influenza vaccination in children with egg allergies have recently changed. Children with only hives following exposure to egg can be vaccinated, as long as injectable influenza vaccine is used as opposed to LAIV, vaccination is by a healthcare provider experienced in recognizing allergic reactions, and the child is observed for 30 minutes following vaccination. Children with more serious allergic reactions to egg, such as angioedema, respiratory symptoms, or anaphylaxis, may be eligible for injectable influenza vaccine, but should be referred to an allergist for an assessment of vaccination risk. Some vaccines (MMR, MMRV, and VAR) contain gelatin, a substance to which persons with known food allergy may develop an anaphylactic reaction. For any persons with a known history of anaphylactic reaction to any component contained in a vaccine, the vaccine package insert should be reviewed and additional consultation sought, such as from a pediatric allergist. Some tips and rubber plungers of vaccine syringes contain latex. These vaccines should not be administered to individuals with a history of severe anaphylactic allergy to latex, but may be administered to people with less severe allergies. Thimerosal is an organic mercurial compound used as a preservative in vaccines since the 1930s. While there is no evidence that thimerosal has caused serious allergic reactions or autism, all routinely recommended vaccines for infants have been manufactured without thimerosal since mid-2001. Thimerosal-free formulations of injectable influenza vaccine are available, and LAIV does not contain thimerosal.
 Other Circumstances
Other Circumstances
Detailed recommendations for preterm low-birth-weight infants; pediatric transplant recipients; Alaskan Natives/American Indians; children in residential institutions or military communities; or refugees, new immigrants, or travelers are available from the CDC (at http://www.cdc.gov/vaccines) and from the AAP’s Red Book.
CDC: General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(RR-02):1 [PMID: 21293327].
Greenhawt MJ et al: Administering influenza vaccine to egg allergic recipients: a focused practice parameter update. Ann Allergy Asthma Immunol 2011;106:11 [PMID: 1195939].
HEPATITIS B VACCINATION
The incidence of reported cases of acute hepatitis B has declined dramatically in the United States, largely attributable to vaccination. Based on surveillance data from 2007, acute hepatitis B incidence has declined by 82% since 1990, to the lowest rate ever measured. The greatest declines have been seen in children younger than 15 years of age, in whom rates have decreased by 98%.
Success in reducing the burden of hepatitis B in the United States is due, in large part, to a comprehensive hepatitis B prevention strategy initiated in 1991. The four central elements of this approach are (1) immunization of all infants beginning at birth; (2) routine screening of all pregnant women for hepatitis B infection, and provision of hepatitis B immune globulin (HBIg) to all infants born to infected mothers; (3) routine vaccination of previously unvaccinated children and adolescents; and (4) vaccination of adults at increased risk of hepatitis B infection.
While high immunization rates have been achieved in young children (more than 91% were fully immunized in 2011), there has been less success in identifying hepatitis B–infected mothers and at immunizing high-risk adults. Of the estimated 23,000 mothers who deliver each year who are hepatitis B surface antigen (HBsAg) positive, only 9000 are identified through prenatal screening. While there is an average of 90 cases of perinatally acquired hepatitis B infection reported to the CDC every year, the actual number of perinatal HBV infections is estimated to be 10–20 times higher than the number currently detected and reported. This circumstance represents a significant missed opportunity for prevention, given that administration of hepatitis B vaccine (HepB) in conjunction with HBIg is 95% effective at preventing mother-to-infant transmission of the virus. Further, many hospitals do not routinely offer HepB to all newborns, despite ACIP recommendations for universal newborn HepB vaccination. Similarly, while HepB alone is 90%–95% effective at preventing hepatitis B infection, only 45% of high-risk adults have been vaccinated.
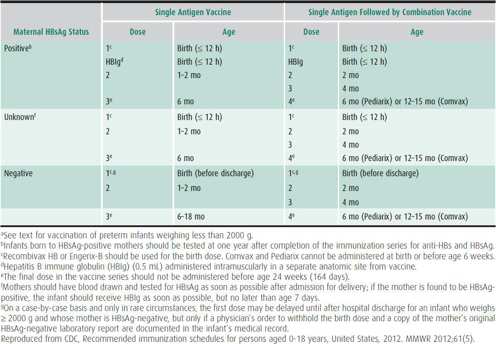
All pregnant women should be routinely screened for HBsAg. Infants born to HBsAg-positive mothers should receive both HepB and HBIg immediately after birth. Infants for whom the maternal HBsAg status is unknown should receive vaccine (but not HBIg) within 12 hours of birth. In such circumstances, the mother’s HBsAg status should be determined as soon as possible during her hospitalization, and the infant given HBIg if the mother is found to be HBsAg positive. For all infants, the hepatitis B immunization series should be started at birth, with the first dose given prior to discharge from the hospital. The ACIP has recommended that any decision to defer the birth dose require an explanation in the medical record, accompanied by a copy of the mother’s negative HBsAg test during the current pregnancy. In 2011, 69% of infants nationally received HepB within 3 days after birth, with wide variation by state (23%–84%).
Routine immunization with three doses of HepB is recommended for all infants and all previously unvaccinated children aged 0–18 years. A two-dose schedule is available for adolescents as well. In addition, persons 19 years and older with an increased risk of exposure to hepatitis B virus should be vaccinated. This includes men who have sex with men, persons with multiple sexual partners, intravenous and injection drug users, recipients of clotting factor concentrates, hemodialysis patients, household contacts and sexual contacts of persons with chronic hepatitis B infection, long-term international travelers to endemic areas, all adults 19 through 59 years of age with type 1 or 2 diabetes mellitus, and all healthcare personnel. HepB is also recommended for persons with chronic liver disease or HIV. Screening for markers of past infection before vaccinating is not indicated for children and adolescents, but may be considered for high-risk adults. Because HepB vaccines consist of an inactivated subunit of the virus, the vaccines are not infectious and are not contraindicated in immunosuppressed individuals or pregnant women.
 Vaccines Available
Vaccines Available
1. Hepatitis B vaccine (Recombivax HB, Merck) contains recombinant HepB only.
2. Hepatitis B vaccine (Engerix-B, GlaxoSmithKline) contains recombinant HepB only.
3. Hepatitis B-Hib (Comvax, Merck) contains vaccines against hepatitis B and Hib.
4. DTaP-HepB-IPV (Pediarix, GlaxoSmithKline) contains vaccines against diphtheria, tetanus, pertussis, hepatitis B, and poliovirus.
Only the noncombination vaccines (Recombivax HB and Engerix-B) can be given between birth and 6 weeks of age. Any single or combination vaccine listed above can be used to complete the hepatitis B vaccination series. Thimerosal has been removed from all pediatric HepB formulations. A combination vaccine against hepatitis A and hepatitis B (Twinrix, GlaxoSmithKline) is available, but is only licensed in the United States for persons 18 years and older.
 Dosage Schedule of Administration
Dosage Schedule of Administration
HepB is recommended for all infants and children in the United States. Table 10–4 presents the vaccination schedule for newborn infants, dependent on maternal HBsAg status. Infants born to mothers with positive or unknown HBsAg status should receive HepB vaccine within 12 hours of birth. Infants born to HBsAg-negative mothers should receive the vaccine prior to hospital discharge.
Table 10–4. Hepatitis B vaccine schedules for newborn infants, by maternal hepatitis B surface antigen (HBsAg) status.a
For children younger than 11 years of age not previously immunized, three intramuscular doses of HepB are needed. Adolescents aged 11–15 years have two options: the standard pediatric three-dose schedule or two doses of adult Recombivax HB (1.0 mL dose), with the second dose administered 4–6 months after the first dose. The vaccine should be given intramuscularly in either the anterolateral thigh or deltoid, depending on the age and size of the patient.
Certain patients may have reduced immune response to HepB vaccination, including preterm infants weighing less than 2000 g at birth, the elderly, immunosuppressed patients, and those receiving dialysis. Preterm infants whose mothers are HBsAg-positive or with unknown HBsAg status should receive both HepB and HBIg within 12 hours of birth. For preterm infants whose mothers are known to be HBsAg-negative, initiation of the vaccination series should be delayed until 30 days of chronologic age if the infant is medically stable or prior to hospital discharge if the infant is discharged before 30 days of age. Pediatric hemodialysis patients and immunocompromised persons may require larger doses or an increased number of doses, with dose amounts and schedules available in the most recent CDC hepatitis B recommendations (see references).
 Contraindications & Precautions
Contraindications & Precautions
HepB should not be given to persons with a serious allergic reaction to yeast or to any vaccine components. Individuals with a history of serious adverse events, such as anaphylaxis, after receiving HepB should not receive additional doses. Vaccination is not contraindicated in persons with a history of Guillain-Barré syndrome, multiple sclerosis, autoimmune disease, other chronic conditions, or in pregnancy.
 Adverse Effects
Adverse Effects
The overall rate of adverse events following vaccination is low. Those reported are minor, including fever (1%–6%) and pain at the injection site (3%–29%). There is no evidence of an association between vaccination and sudden infant death syndrome, multiple sclerosis, autoimmune disease, or chronic fatigue syndrome.
 Postexposure Prophylaxis
Postexposure Prophylaxis
Postexposure prophylaxis is indicated for unvaccinated persons with perinatal, sexual, household, percutaneous, or mucosal exposure to hepatitis B virus. When prophylaxis is indicated, unvaccinated individuals should receive HBIg (0.06 mL/kg) and the first dose of HepB at a separate anatomic site. For sexual contact or household blood exposure to an acute case of hepatitis B, HBIg and HepB should be given. Sexual and household contacts of someone with chronic infection should receive HepB (but not HBIg). For individuals with percutaneous or permucosal exposure to blood, HepB should be given, and HBIg considered depending on the HBsAg status of the person who was the source of the blood and on the vaccination response status of the exposed person. All previously vaccinated persons exposed to hepatitis B should be retested for anti-HBs. If antibody levels are adequate (≥ 10 mIU/mL), no treatment is necessary. If levels are inadequate and the exposure was to HBsAg-positive blood, HBIg and vaccination are required.
 Antibody Preparations
Antibody Preparations
HBIg is prepared from HIV-negative and hepatitis C virus-negative donors with high titers of hepatitis B surface antibody. The process used to prepare this product inactivates or eliminates any undetected HIV and hepatitis C virus.
CDC: A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States, part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(RR-16):1 [PMID: 16371945].
Wasley A et al: The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis 2010;202:192 [PMID: 20533878].
Willis BC et al: Gaps in hospital policies and practices to prevent perinatal transmission of hepatitis B virus. Pediatrics 2010;125:704 [PMID: 20211952].
ROTAVIRUS VACCINATION
Rotavirus is the leading cause of hospitalization and death from acute gastroenteritis in young children worldwide. The burden of rotavirus is particularly severe in the developing world, where as many as 500,000 children die each year from rotavirus-associated dehydration and other complications. While deaths from rotavirus were uncommon in the United States (20–60 deaths per year), prior to the introduction of rotavirus vaccine, rotavirus infections caused substantial morbidity annually with an estimated 2.7 million diarrheal illnesses, 410,000 office visits, and 55,000–70,000 hospitalizations.
A rhesus-based rotavirus vaccine (RotaShield, Wyeth-Lederle) was licensed by the FDA and recommended for routine use by the ACIP in 1998 but was withdrawn from the market within one year after it was noted that there was an increased risk of intussusception after the first dose. Two other rotavirus vaccines were in development at the time, and underwent extensive prelicensure testing. No association with intussusception was found in prelicensure studies for either new rotavirus vaccine. The ACIP made a recommendation to include pentavalent rotavirus vaccine (RV5; RotaTeq) in the routine infant series in February 2006, a recommendation that was updated in June 2008 to include the monovalent rotavirus vaccine (RV1; Rotarix).
Since the introduction of these vaccines, their use has increased steadily. Vaccination coverage in 2011 in the United States for ≥ 2 doses of rotavirus vaccine was 67%, up from 44% just two years earlier. The impact of the vaccines has been substantial, reducing both hospitalizations and outpatient visits. Death from rotavirus disease was a rare occurrence in the United States prior to licensure, but rotavirus vaccine has had a profound impact on deaths in the developing countries where it has been introduced.
However, there were two recent findings that raised concern about the safety of the newer rotavirus vaccines. In March 2010, the FDA recommended temporarily suspending use of RV1 due to the detection of porcine circovirus, a nonhuman pathogen, in the vaccine. This recommendation was lifted 2 months later after investigations showed that there was likely no threat to human health. Shortly thereafter, in August 2010, the Global Advisory Committee on Vaccine Safety of the World Health Organization reviewed preliminary data from postmarketing studies that showed a possible increased risk of intussusception for RV1 in Mexico. On September 22, 2010, the FDA recommended a label change for RV1 advising providers of the new data.
 Vaccines Available
Vaccines Available
1. RV5 (Rotateq, Merck) is a pentavalent, live, oral, human-bovine reassortant rotavirus vaccine. The vaccine is a liquid, does not require any reconstitution, and does not contain any preservatives. The dosing tube is latex-free.
2. RV1 (Rotarix, GlaxoSmithKline) is a monovalent, live, oral, attenuated human rotavirus vaccine. The vaccine needs to be reconstituted with 1 mL of diluent using a prefilled oral applicator. The vaccine does not contain any preservatives. The oral applicator contains latex.
 Dosage & Schedule of Administration
Dosage & Schedule of Administration
Either RV5 or RV1 can be used to prevent rotavirus gastroenteritis. RV5 should be administered orally, as a three-dose series, at 2, 4, and 6 months of age. RV1 should be administered orally, as a two-dose series, at 2 and 4 months of age. For both rotavirus vaccines, the minimum age for dose 1 is 6 weeks, and the maximum age for dose 1 is 14 weeks and 6 days. The vaccination series should not be started at 15 weeks of age or older, because of the lack of safety data around administering dose 1 to older infants. The minimum interval between doses is 4 weeks. All doses should be administered by 8 months and 0 days of age. While the ACIP recommends that the vaccine series be completed with the same product (RV5 or RV1) used for the initial dose, if this is not possible, providers should complete the series with whichever product is available.
Either rotavirus vaccine can be given simultaneously with all other recommended infant vaccines. Rotavirus vaccine can be given to infants with minor acute illness. No restrictions are placed on infant feeding before or after receiving rotavirus vaccine. Infants readily swallow the vaccine in most circumstances; however, if an infant spits up or vomits after a dose is administered, the dose should not be readministered; the infant can receive the remaining doses at the normal intervals.
 Contraindications & Precautions
Contraindications & Precautions
Rotavirus vaccine should not be given to infants with a severe hypersensitivity to any components of the vaccine, to infants who had a serious allergic reaction to a previous dose of the vaccine, or to infants with a history of intussusception from any cause. Because the RV1 oral applicator contains latex rubber, RV1 should not be given to infants with a severe latex allergy; RV5 is latex-free. Both vaccines are contraindicated in infants with severe combined immunodeficiency (SCID). Vaccination should be deferred in infants with acute moderate or severe gastroenteritis. Limited data suggest that rotavirus vaccination is safe and effective in premature infants. A small trial in South Africa also demonstrated that RV1 was well tolerated and immunogenic in HIV-infected children. However, vaccine safety and efficacy in infants with immunocompromising conditions other than SCID, preexisting chronic gastrointestinal conditions (eg, Hirschsprung disease or short-gut syndrome), or a prior episode of intussusception, has not been established. Clinicians should weigh the potential risks and benefits of vaccination in such circumstances. Infants living in households with pregnant women or immunocompromised persons can be vaccinated.
 Adverse Effects
Adverse Effects
Because of new information regarding a possible increased risk of intussusception after the first dose from postmarketing surveillance done in Mexico, the FDA recently recommended a change to the labeling of RV1 (Rotarix), but not RV5 (Rotateq), informing providers of the possible increased risk.
Data from a large study in Mexico and Brazil found that there may be a small increase in the risk of intussusception in the 1–7 day time frame after the first dose of RV1 in Mexico (incident rate ratio 5.3, 95% confidence interval [CI] 3.0–9.3), but not in Brazil (incident rate ratio 1:1, 95% CI 0.3–3.3). A possible explanation for the discrepancy is that live oral poliovirus vaccine is given in Brazil, but not in Mexico, where inactivated poliovirus vaccine is given. It is important to note that the same study showed that the benefits of rotavirus vaccination far exceeded any possible risk, and recommendations for use of RV1 have not changed in Mexico or elsewhere. Also, the background rate of intussusception in Mexico, between 60 and 90 per 100,000 children per year, is higher than in the United States. Active surveillance conducted in the United States has not shown an increased risk of intussusception following RV5 vaccination; comparable data are not yet available for RV1 vaccination.
CDC: Prevention of rotavirus gastroenteritis among infants and children. MMWR Recomm Rep 2009;58(RR-2):1 [PMID: 19194371].
CDC. Addition of history of intussusception as a contraindication for rotavirus vaccination. MMWR 2011;60:1427 [PMID: 22012117].
Patel MM et al: Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 2011;364:2283 [PMID: 21675888].
Staat MA et al: Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 2011;128:e267 [PMID: 21768317].
Yen C et al: Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics 2011;127:e9 [PMID: 21172995].
DIPHTHERIA-TETANUS-ACELLULAR PERTUSSIS VACCINATION
Diphtheria, tetanus, and pertussis (DTP) vaccines have been given in a combined vaccine for many decades, and have dramatically reduced each of these diseases. The efficacy with antigens in the combined vaccine is similar to that with antigens in single component vaccines. The pertussis component of DTP vaccines contains whole-cell pertussis antigens. Although these vaccines are used widely in the world, DTP vaccines have been entirely replaced in the United States with DTaP vaccines, which are acellular pertussis vaccines made with purified, inactivated components of the bacterium.
Diphtheria is caused by a gram-positive bacillus, Corynebacterium diphtheriae. It is a toxin-mediated disease, with diphtheria toxin causing local tissue destruction, as in pharyngeal and tonsillar diphtheria, as well as systemic disease, particularly myocarditis and neuritis. The overall case fatality rate is between 5% and 10%, with higher death rates in persons younger than 5 years, or older than 40 years of age. As many as 200,000 cases of diphtheria occurred each year in the 1920s in the United States. Largely because of successful vaccination programs, only five cases of diphtheria have been reported in the United States since 2000, and a confirmed case has not been reported since 2003. In the last several decades, the majority of diphtheria cases in the United States have been in unimmunized or inadequately immunized persons. The clinical efficacy of diphtheria vaccine is not precisely known, but has been estimated to be greater than 95%.
The anaerobic gram-positive rod Clostridium tetani causes tetanus, usually through infection of a contaminated wound. When C tetani colonizes devitalized tissue, the exotoxin tetanospasmin is disseminated to inhibitory motor neurons, resulting in generalized rigidity and spasms of skeletal muscles. Tetanus-prone wounds include (1) puncture wounds, including those acquired due to body piercing, tattooing, and intravenous drug abuse; (2) animal bites; (3) lacerations and abrasions; and (4) wounds resulting from nonsterile neonatal delivery and umbilical cord care (neonatal tetanus). In persons who have completed the primary vaccination series and have received a booster dose within the past 10 years, vaccination is virtually 100% protective. In 2010, 26 cases of tetanus occurred in the United States with almost all cases in persons who have had inadequate, distant (> 10 years) or no tetanus immunization.
Pertussis is also primarily a toxin-mediated disease. Called whooping cough because of the high-pitched inspiratory whoop that can follow intense paroxysms of cough, pertussis is caused by the bacterium Bordetella pertussis. Complications from pertussis include death, often from associated pneumonia, seizures, and encephalopathy. Prior to the widespread use of pertussis vaccines in the 1940s, roughly 1 million pertussis cases were reported over a 6-year period. Pertussis incidence in the United States declined dramatically between the 1940s and 1980s, but beginning in the early 1980s, incidence has been slowly increasing, with adolescents and adults accounting for a greater proportion of reported cases. Reasons for increased incidence include improved detection of cases with better laboratory testing methodology (polymerase chain reaction, serology), improved recognition of cases in adolescents and adults, and waning protection from childhood vaccination or prior infection. Infants less than 6 months of age have the highest rate of pertussis infection (143 cases per 100,000) and greater than 90% of pertussis deaths occur in neonates and infants less than 3 months of age.
In 2010, 27,550 cases of pertussis were reported in the United States with many localized outbreaks necessitating enhanced vaccination programs. California had the highest reported incidence since 1958 with a rate of 26.0 cases/100,000; over 9143 cases; and 10 infant deaths. A single booster dose of a different formulation, Tdap, is now recommended for all adolescents and adults, as is discussed in more detail later in this chapter. Providing a booster dose of pertussis-containing vaccine may prevent adolescent and adult pertussis cases, and also has the potential to reduce the spread of pertussis to infants, who are most susceptible to complications from the disease. Currently the ACIP is considering expanding recommendations to include regular booster doses of Tdap in an attempt to protect infants and mitigate current pertussis outbreaks.
 Vaccines Available
Vaccines Available
Diphtheria, Tetanus, and Acellular Pertussis Combinations
1. DTaP (Daptacel, sanofi pasteur; Infanrix, GlaxoSmithKline) contains tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccine. This DTaP is licensed for ages 6 weeks through 6 years and can be used for doses 1 through 5.
2. Tdap (Boostrix, GlaxoSmithKline) is a tetanus-reduced dose diphtheria-acellular pertussis vaccine formulated for persons 10 years of age and older, including adults and the elderly.
3. Tdap (Adacel, sanofi pasteur) is a tetanus-diphtheria-acellular pertussis vaccine approved for persons 11 through 64 years of age.
DTaP Combined With Other Vaccines
1. DTaP-IPV-Hepatitis B (Pediarix, GlaxoSmithKline) contains DTaP combined with poliovirus and HepB vaccines. It is approved for the first three doses of the DTaP and IPV series, given at 2, 4, and 6 months of age. Although it is approved for use through age 6 years, it is not licensed for booster doses. It cannot be used, for example, as the fourth dose of DTaP (the dose typically given at 15–18 months of age).
2. DTaP-IPV-Hib (Pentacel, sanofi pasteur) contains DTaP, IPV, and Hib vaccines. The Hib component is Hib capsular polysaccharide bound to tetanus toxoid. This vaccine is approved for use as doses 1 through 4 of the DTaP series among children 6 weeks to 4 years of age. It is typically given at 2, 4, 6, and 15–18 months of age, and should not be used as the fifth dose in the DTaP series.
3. DTaP-IPV (Kinrix, GlaxoSmithKline) contains DTaP and IPV vaccines. The vaccine is licensed for children 4–6 years of age, for use as the fifth dose of the DTaP vaccine series and the fourth dose of the IPV series. Using this vaccine would reduce by one the number of injections a 4- to 6-year-old child would receive.
Diphtheria and Tetanus Combinations
1. DT (generic, sanofi pasteur) contains tetanus toxoid and diphtheria toxoid to be used only in children younger than age 7 years with a contraindication to pertussis vaccination.
2. Td (Decavac, sanofi pasteur; generic, Massachusetts Biological Labs) contains tetanus toxoid and a reduced quantity of diphtheria toxoid, which is typically used for adults requiring tetanus prophylaxis.
Tetanus Only
TT (generic, sanofi pasteur) contains tetanus toxoid only, and can be used for adults or children. However, the use of this single-antigen vaccine is generally not recommended, because of the need for periodic boosting for both diphtheria and tetanus.
 Dosage & Schedule of Administration
Dosage & Schedule of Administration
Although several different vaccines are available, a few general considerations can help guide their use in specific circumstances. DTaP (alone or combined with other vaccines) is used for infants and children between 6 weeks and 6 years of age. Children 7–10 years of age not fully immunized against pertussis (meaning those who have not received five prior doses of DTaP, or four doses of DTaP if the fourth dose was given on or after the fourth birthday), who have no contraindications to pertussis immunization, should receive a single dose of Tdap for pertussis protection. For adolescents and adults, a single dose of Tdap is used, followed by booster doses of Td every 10 years; a detailed description of Tdap use is provided later in this chapter.
The primary series of DTaP vaccination should consist of four doses, given at 2, 4, 6, and 15–18 months of age. The fourth dose may be given as early as 12 months of age if 6 months have elapsed since the third dose. Giving the fourth dose between 12 and 15 months of age is indicated if the provider thinks the child is unlikely to return for a clinic visit between 15 and 18 months of age. Children should receive a fifth dose of DTaP at 4–6 years of age. However, a fifth dose of DTaP is not needed if the fourth dose was given after the child’s fourth birthday. The same brand of DTaP should be used for all doses if feasible.
 Contraindications & Precautions
Contraindications & Precautions
DTaP vaccines should not be used in individuals who have had an anaphylactic-type reaction to a previous vaccine dose or to a vaccine component. DTaP should not be given to children who developed encephalopathy, not attributable to another identified cause, within 7 days of a previous dose of DTaP or DTP. DTaP vaccination should also be deferred in individuals with progressive neurologic disorders, such as infantile spasms, uncontrolled epilepsy, or progressive encephalopathy, until their neurologic status is clarified and stabilized.
Precautions to DTaP vaccination include: high fever (≥ 40.5°F), persistent inconsolable crying, or shock-like state within 48 hours of a previous dose of DTP or DTaP; seizures within 3 days of a previous dose of DTP or DTaP; Guillain-Barré syndrome less than 6 weeks after a previous tetanus-containing vaccine; or incident moderate or severe acute illness with or without a fever.
 Adverse Effects
Adverse Effects
Local reactions, fever, and other mild systemic effects occur with acellular pertussis vaccines at one-fourth to two-thirds the frequency noted following whole-cell DTP vaccination. Moderate to severe systemic effects, including fever of 40.5°C, persistent inconsolable crying lasting 3 hours or more, and hypotonic-hyporesponsive episodes, are less frequent than with whole-cell DTP. These are without sequelae. Severe neurologic effects have not been temporally associated with DTaP vaccines in use in the United States. A recent study from Canada showed no evidence of encephalopathy related to pertussis vaccine (< 1 case per 3 million doses of DTP and < 1 per 3.5 million doses of DTaP). Data are limited regarding differences in reactogenicity among currently licensed DTaP vaccines. With all currently licensed DTaP vaccines, reports of substantial local reactions at injection sites have increased with increasing dose number (including swelling of the thigh or entire upper arm after receipt of the fourth and fifth doses).
 Diphtheria Antibody Preparations
Diphtheria Antibody Preparations
Diphtheria antitoxin is manufactured in horses. Dosage depends on the size and location of the diphtheritic membrane and an estimate of the patient’s level of intoxication. Sensitivity to diphtheria antitoxin must be tested before it is given. Consultation on the use of diphtheria antitoxin is available from the CDC’s National Center for Immunization and Respiratory Diseases. Diphtheria antitoxin is not commercially available in the United States and must be obtained from the CDC.
 Tetanus Antibody Preparations
Tetanus Antibody Preparations
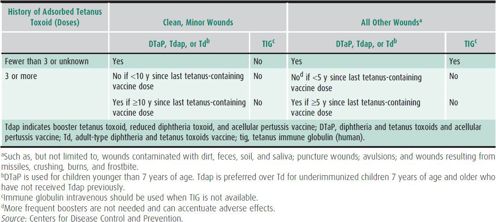
Human tetanus immune globulin (TIg) is indicated in the management of tetanus-prone wounds in individuals who have had an uncertain number or fewer than three tetanus immunizations. Persons fully immunized with at least three doses do not require TIg, regardless of the nature of their wounds (Table 10–5). The optimal dose of TIg has not been established, but some sources recommend 3000–6000 units as a single dose, with part of the dose infiltrated around the wound.
Table 10–5. Guide to tetanus prophylaxis in routine wound management.
CDC: Updated recommendations for the use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2011. MMWR 2011;60:13 [PMID: 21228763].
CDC: Diphtheria. In: Atkinson W et al (eds): Epidemiology and Prevention of Vaccine-Preventable Diseases, 12th ed., second printing. Public Health Foundation; 2012:75.
CDC: Pertussis. In: Atkinson W et al (eds): Epidemiology and Prevention of Vaccine-Preventable Diseases, 12th ed., second printing. Public Health Foundation; 2012:215.
CDC: Tetanus. In: Atkinson W et al (eds): Epidemiology and Prevention of Vaccine–Preventable Diseases, 12th ed., second printing. Public Health Foundation; 2012:291.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


