Hypospadias
Chordee, which is ventral curvature of the penis, has an inconsistent association with hypospadias. The degree of chordee is ultimately more significant in the surgical treatment of hypospadias than is the initial location of the meatus. A subcoronal hypospadias with little or no chordee is much less complicated to repair than is one with significant chordee and insufficient ventral skin. For this reason, when discussing the degrees of hypospadias, it is more appropriate to use the clinically relevant and common classification system that refers to the meatal location after the chordee has been released (Box 59-1).1
Embryology
Normal phallic development occurs in weeks 7 to 14 of gestation. By 6 weeks of gestation, the genital tubercle is formed anterior to the urogenital sinus. In the next week, two genital folds form caudad to the tubercle and a urethral plate develops between them. Under the influence of testosterone from the fetal testes, which begins to be produced at about 8 weeks of gestation, the inner genital folds fuse medially to create a tube that communicates with the urogenital sinus and runs distally to end at the base of the glans. The formation of the penile urethra is thus generally completed by the end of the first trimester.2
Classically, the glanular urethra is thought to form as an ectodermal ingrowth on the glans. This ingrowth deepens to meet the distal urethra that has formed from the closure of the endodermal genital folds. The capacious junction of these two structures is the fossa navicularis.3 Recently, this theory has been challenged by the endodermal ingrowth theory. It suggests that the entire urethra forms from the urogenital sinus, which is endoderm. This endoderm differentiates into stratified squamous epithelium.4 The formation of the glanular urethra is the last step in the formation of the completed urethra. This sequence probably accounts for the predominance of glanular and coronal hypospadias.
Historical Perspective
The first description of hypospadias and its operative correction was reported in the 1st and 2nd centuries by the Alexandrian surgeons Heliodorus and Antyllus. They described the defect of hypospadias and its relation to problems with urination and ineffective coitus. They further described a surgical treatment consisting of amputation of the glans distal to the hypospadiac meatus.5,6
Little progress was made in the surgical treatment of hypospadias until the 19th century, when two Americans, Mettauer and Bush, described using a trocar to establish a channel from the meatus to the glans. Dieffenbach also described a similar technique in the 1830s. None of these methods was very successful.5
In 1874, Theophile Anger reported the successful repair of a penoscrotal hypospadias using the technique described in 1869 by Thiersch for the repair of epispadias in which lateral skin flaps were tubularized to form the neourethra. Anger’s report initiated the modern era of hypospadias surgery characterized by the use of local skin flaps.7,8 Duplay soon described his two-stage technique.6 In the first stage, the chordee was released; in the second stage, a ventral midline strip of skin was covered by closure of the lateral penile skin flaps in the midline. Duplay did not believe that it was necessary to form the urethral tube completely because he thought that epithelialization would occur even if an incomplete tube was buried under the lateral skin flaps. Browne used this concept in his well-known ‘buried strip’ technique, which was widely used in the early 1950s.9
In the late 1800s, various surgeons reported on penile, scrotal, and preputial flap techniques for multistage procedures. Several of them used the technique of burying the penis in the scrotum to obtain skin coverage, similar to the technique described by Cecil and Cuip in the late 1950s.10 In 1913, Edmonds was the first to describe the transfer of preputial skin to the ventrum of the penis at the time of release of chordee. At a second stage, the Duplay tube was created to complete the urethral closure. Byars popularized this two-stage technique in the early 1950s.11 Smith further improved the outcomes by denuding the epithelium of one of the lateral skin flaps to give a ‘pants-over-vest’ closure to reduce the risk of fistula formation.12 Belt devised another preputial transfer, two-stage procedure that was popularized by Fuqua in the 1960s.13
Nove-Josserand, in 1897, was the first to report the use of a free, split-thickness skin graft in an attempt to repair hypospadias.14 Over the next 20 years, various other tissues were used as free grafts, including saphenous vein, ureter, and appendix, with varying success. McCormack used a free, full-thickness skin graft in a two-stage repair.15 In 1941, Humby described a one-stage approach using the full thickness of the foreskin.16 Devine and Horton later popularized this free preputial graft technique with very good results.17
In 1947, Memmelaar described the use of bladder mucosa as a free graft technique in a one-stage repair.18 In 1955, Marshall and Spellman used bladder mucosa in a two-stage technique.19 Urologists in China also experienced good success with a primary repair using bladder mucosa. This technique was developed independently during the period of scientific and cultural isolation in China.20 Buccal mucosa from the lip was employed for urethral reconstruction in 1941 by Humby16 and has recently gained renewed attention as a free graft technique.21
Improvement in preputial and meatal-based vascularized flaps over the last 30 to 40 years have greatly advanced hypospadias repair. Through contributions of surgeons such as Mathieu, Barcat,1 Mustardé,22 Broadbent,23 Hodgson,24 Horton and Devine,17 Standoli,25 and Duckett,26 the single-stage repair of even the most severe forms of hypospadias has become commonplace.
Clinical Aspects
Incidence
The incidence of hypospadias has been estimated between 0.8 and 8.2 per 1,000 live male births.27 The wide variation probably represents some geographic and racial differences, but of more significance is the exclusion of the more minor degrees of hypospadias in some reports. If all degrees of hypospadias, even the most minor, are included, then the incidence is probably 1 in 125 live male births.28 With the most quoted figure of 1 per 250 live male births, it can be assumed that more than 6,000 boys are born with hypospadias each year in the USA.29
Etiology
A defect in the androgen stimulation of the developing penis, which precludes complete formation of the urethra and its surrounding structures, is the ultimate cause of hypospadias. This defect can occur from deficient androgen production by the testes and placenta, from failure of testosterone to convert to dihydrotestosterone by the 5α-reductase enzyme, or from deficient androgen receptors in the penis. Various disorders of sexual differentiation (DSD) can cause deficiencies at any point along the androgen-stimulation axis. These are discussed in Chapter 62.
The origin of hypospadias not associated with DSD is unclear. An endocrine cause has been implicated in some reports that show a diminished response to human chorionic gonadotropin (hCG) in some patients with hypospadias, suggesting delayed maturation of the hypothalamic–pituitary axis.30,31 Other reports have described an increased incidence of hypospadias in monozygotic twins, suggesting an insufficient amount of hCG production by the single placenta to accommodate the two male fetuses.32
Environmental causes also have been implicated. A higher incidence of hypospadias has been noted in winter conceptions.32 A weak association between hypospadias and the maternal ingestion of progestin-like agents has also been noted.33,34 No association has been found between hypospadias and oral contraceptive use before or during early pregnancy.35
Genetic factors in the etiology of hypospadias are implicated by the higher incidence of this anomaly in first-degree relatives of hypospadiac patients.27,34,36 In one study that evaluated 307 families, the risk of hypospadias in a second male sibling was 12%. If the index child and his father were affected, the risk for a second sibling increased to 26%. If the index child and a second-degree relative (rather than the father) were affected, the risk of the sibling being affected was only 19%.36 This pattern suggests a multifactorial mode of inheritance, with these families having a higher than average number of influential genes for creation of hypospadias.36 A combination of the endocrine, environmental, and genetic factors likely determines the potential for developing the hypospadias complex in any one individual.31,32
Anatomy of the Defect
The clinical significance of hypospadias is related to several factors. The abnormal location of the meatus and the tendency toward meatal stenosis result in a ventrally deflected and splayed stream. This fact makes the stream difficult to control and often makes it difficult for the patient to void while standing. The ventral curvature associated with chordee can lead to painful erections, especially with severe chordee. Impaired copulation and thus inadequate insemination is a further consequence of significant chordee. In addition, the unusual cosmetic appearance associated with the hooded foreskin, flattened glans, and ventral skin deficiency frequently has an adverse effect on the psychosexual development of the adolescent with hypospadias.37–41 All of these factors are evidence that early surgical correction should be offered to all boys with hypospadias, regardless of the severity of the defect.
The distal form of hypospadias is the most common (see Box 59-1). Frequently, little or no associated chordee is present (Fig. 59-1). The size of the meatus and the quality of the surrounding supportive tissue as well as the configuration of the glans are variable, and ultimately determine the appropriate operative technique. Well-formed, mobile perimeatal skin and a deep ventral glans groove may allow development of perimeatal flaps to create the urethra (Fig. 59-2). In contrast, atrophic and immobile skin around the meatus may require tissue transfer from the preputium to form the neourethra.
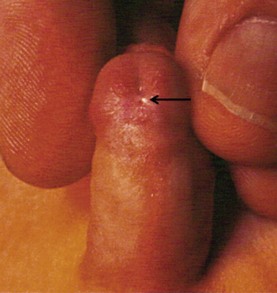
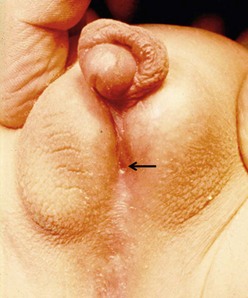
FIGURE 59-1 Distal hypospadias with a stenotic meatus (arrow) located on the glans with no chordee. This patient is a good candidate for a meatal advancement and glanuloplasty (MAGPI) procedure or a tubularized incised urethral plate (TIP) operation.
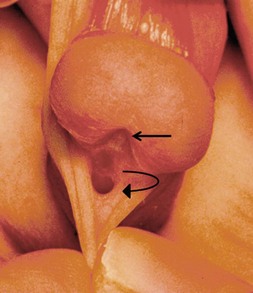
FIGURE 59-2 Patulous, subcoronal meatus (curved arrow) with mobile perimeatal skin and deep ventral glans groove (straight arrow). This is a good variant for a meatal-based flap procedure, glans approximation (GAP), or tubularized incised urethral plate (TIP) repair.
An unusual variant of the distal hypospadias is the large wide-mouthed meatus with a circumferential foreskin (the megameatus/intact prepuce variant) (Fig. 59-3).42 Owing to the intact prepuce, this variant is often not identified until a circumcision has been performed. If clinicians discover hypospadias during circumcision, they should stop and preserve the foreskin, even if the dorsal slit has been created.
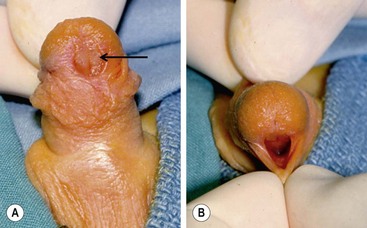
FIGURE 59-3 (A) Previously circumcised penis with megameatus (arrow) and intact circumferential prepuce. (B) Large wide-mouthed meatus in the same penis as shown in (A).
At times, the distally located meatus may be associated with significant chordee, sometimes of a severe degree (Fig. 59-4). The release of the chordee places the meatus in a much more proximal location, requiring more complicated transfers of tissue to bridge the gap between the proximal meatus and the tip of the glans.
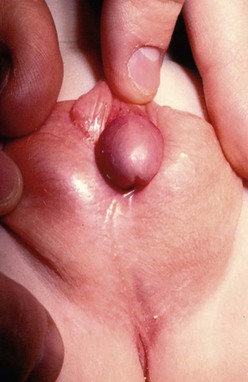
FIGURE 59-4 Scrotal hypospadias with severe chordee and marked penoscrotal transposition is seen in this infant.
When the meatus is located on the penile shaft, the character of the urethral plate (midline ventral shaft skin distal to the meatus) is important in determining what type of repair is possible. A well-developed and elastic urethral plate suggests minimal, if any, distal ventral curvature (Fig. 59-5). However, a thin atrophic urethral plate heralds a significant chordee. The proximal supportive tissue of the urethra also is important. If there is a lack of spongiosum proximal to the hypospadiac meatus, this portion of the native urethra is not substantial enough to use in the repair (Fig. 59-6). Therefore, the neourethra must be constructed from the point of adequate spongiosum.
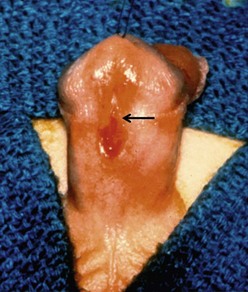
FIGURE 59-5 Midshaft hypospadias with elastic, well-developed urethral plate distal to the meatus (arrow). No significant chordee is present. This is a good variant for an onlay island flap procedure or possibly a tubularized incised urethral plate operation.
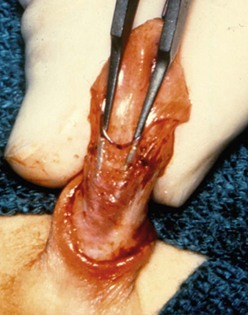
FIGURE 59-6 Midshaft hypospadias with a lack of spongiosum support proximal to the meatus. The urethra should be opened back to an area of good spongiosum support at the penoscrotal junction.
The position of the meatus at the penoscrotal, scrotal, or perineal location is usually associated with severe chordee, which requires chordee release and an extensive urethroplasty (Fig. 59-7). This type is usually more predictable in the preoperative period as to the choice of technique than are some of the more distal types previously discussed.
Associated Anomalies
Inguinal hernia and undescended testes are the most common anomalies associated with hypospadias. They occur in 7–13% of patients with a greater incidence when the meatus is more proximal.43–45 An enlarged prostatic utricle also is more common in posterior hypospadias, with an incidence of about 11%.44 Infection is the most common complication of a utricle, but surgical excision is rarely necessary.46 Several reports have emphasized significantly high numbers of upper urinary tract anomalies associated with hypospadias,47–50 suggesting that routine upper tract screening is necessary. However, when the association is studied selectively, it is evident that the types of hypospadias at risk for significant upper tract anomalies are the penoscrotal and perineal forms, and those associated with other organ system abnormalities.43,45 When one, two, or three other organ system abnormalities also occur, the incidence of significant upper tract anomalies is 7%, 13%, and 37%, respectively. Associated myelomeningocele and imperforate anus carry a 33% and 46% incidence, respectively, of upper urinary tract malformations. In isolated posterior hypospadias, the incidence of associated upper tract anomalies is 5%.45
In middle and distal hypospadias, when not associated with other organ system anomalies, the incidence is similar to that in the general population.43,45,51 Therefore, it is recommended that screening for upper urinary tract abnormalities by voiding cystourethrogram and renal ultrasonography be performed in patients with penoscrotal and perineal forms of hypospadias, and in those with anomalies associated with at least one additional organ system. Screening should also be done in patients with other known indications, such as a history of urinary tract infection, upper or lower tract obstructive symptoms, hematuria, and in those boys having a strong family history of urinary tract abnormalities.52
DSD are also potentially associated with hypospadias. This association is rare in the routine forms of hypospadias. Failure of testicular descent, micropenis, penoscrotal transposition (see Fig. 59-4), or bifid scrotum (see Fig. 59-7) when associated with hypospadias, are all signs of potential DSD and warrant evaluation with karyotype screening.27,53,54
Treatment
Age at Repair
The technical advances over the past few decades have made it possible to repair hypospadias in the first 6 months of life in most patients.55–57 Some surgeons have suggested delaying repair until after the child is age 2 years.52,58–60 However, most surgeons who deal routinely with hypospadias prefer to perform the repair when the patient is 6 to 12 months old.53,54,56,57,61,62 One study compared the emotional, psychosexual, cognitive, and operative risks for hypospadias. The ‘optimal window’ recommended for repair was age 6 to 15 months.63 There is also evidence that healing may be better with decreased inflammatory factors and less scarring in the less than 6 month age group.62 Unless other health or social problems require delay, we believe the ideal time to complete penile reconstruction in the pediatric patient is about age 5 to 6 months.64 The anesthetic risk is low and, at this age, postoperative care is much easier for the parents than it is when the child is a toddler.
Objectives of Repair
The objectives of hypospadias correction are divided into the following categories:
1. Complete straightening of the penis
2. Locating the meatus at the tip of the glans
3. Forming a symmetric, conically shaped glans
4. Constructing a neourethra uniform in caliber
If these objectives can all be attained, the ultimate goal of forming a ‘normal’ penis for the child with hypospadias can be accomplished.
Straightening
Curvature of the penis is difficult to judge, at times, in the preoperative period. Artificial erection, by injecting physiologic saline in the corpora at the time of operation allows determination of the exact degree of curvature.65 This curvature may be caused only by ventral skin or subcutaneous tissue tethering, which is corrected with the release of the skin and dartos layer.66,67 Infrequently, the curvature may be secondary to true fibrous chordee, which requires division of the urethral plate and excision of the fibrous tissue down to the tunica albuginea.
Sometimes, even after extensive ventral dissection of chordee tissue, a repeated artificial erection still reveals the presence of significant ventral curvature. This finding is usually secondary to the uncommon problem of corporal body disproportion which is caused by a true deficiency of ventral corporal development. This problem can be corrected by making a releasing incision in the ventral tunica albuginea and inserting either a dermal or a tunica vaginalis patch to expand the deficient ventral surface.68,69 Others have suggested the use of small intestinal submucosa as an off-the-shelf substitute for the autologous grafts.70 Another technique is to excise wedges of tunica albuginea dorsally with transverse closure to shorten this dorsal surface and straighten the penis.71,72 Other surgeons have had success with dorsal plication without excision of tunica albuginea.73,74 Anatomic studies suggest that this plication should be done in the midline dorsally.75 Still others advocate corporal rotation dorsally with or without penile disassembly to correct severe chordee.76,77
Axial rotation of the penis, or penile torsion, is another aspect of penis straightening that must be managed. This problem can generally be corrected by releasing the dartos layer as far proximal as possible on the penile shaft. This allows the ventral shaft to rotate back to the midline and corrects the torsion. Chordee or torsion can also occur without hypospadias (Fig. 59-8). The management of these boys encompasses the same spectrum of approaches as for hypospadias.78,79
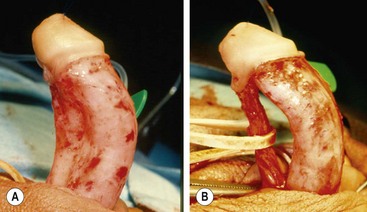
FIGURE 59-8 (A) Chordee without hypospadias. The meatus is located at the tip of the glans with marked ventral curvature. (B) Fibrous ventral tissue is all released. The urethra is mobilized, but curvature persists, indicating corporeal body disproportion. This requires a ventral patch or dorsal plication (see text).
Locating the Meatus
In glanular and subcoronal variants, the configuration of the meatus is the factor that determines what technique is used to move the meatus distally on the glans. Meatoplasty with or without dorsal advancement, distal urethral mobilization and tubularization, or meatal-based flaps are the methods selected in most cases of distal hypospadias.80,81 In the more proximal forms, creating the neourethra with local vascularized skin flaps or free grafts allows positioning the urethra at the end of the penis. Alternatively, glans channeling or glans splitting allows creation of the meatus at the tip of the glans.1,17,22,26,82,83