Human Papillomavirus Infection and Cervical Cancer Screening and Prevention in Adolescents
Sarah Feldman
Jessica A. Kahn
Paula A. Hillard
Background
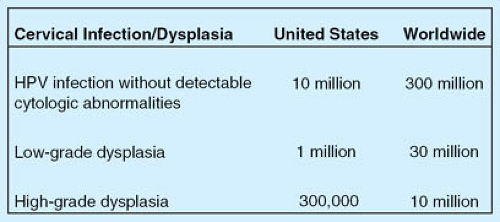
Human papillomavirus (HPV) infection is very common both in the United States and worldwide (1,2). Most HPV infections become undetectable over time and cause few to no clinical sequelae. Certain subtypes of HPV (“high-risk HPV”), however, are more likely to persist and cause cellular changes associated with high-grade cervical precancers and cancers. These changes can be detected and treated. Ideally, therefore, cervical cancer should be preventable: it is one of the few cancers for which the etiology is understood (infection with a high-risk HPV subtype), there is a long time from infection until development of precancerous and then cancerous changes, and we have effective means for detecting and treating precancers. However, rates of cervical cancer and cervical cancer mortality vary tremendously among countries (see Figs. 20-1 and 20-2 for rates of cervical cancer, cervical dysplasia, and HPV infection in the United States and worldwide).
In more developed countries, cervical cancer is relatively rare, due to aggressive programs of screening and early intervention. In contrast, in less developed countries, cervical cancer remains the second highest cause of mortality due to cancer among women (1). Reasons for this disparity include the cost and expertise required for successful cytologic screening, as well as the limited resources available for treatment of precancers and early-stage disease.
Certain HPV types known as “low risk” types may cause low-grade dysplasia, condyloma acuminata, or anogenital warts. Genital warts (condyloma) are very common and may have significant effects on quality of life due to physical discomfort (itching, bleeding, distortion of anatomy) and may cause psychological distress, but they are not precancerous. In patients with impaired cellular immunity, genital warts may be a significant cause of morbidity, due to the symptoms of the disease, its failure to clear on its own, and sequelae of treatment. In areas of the world with a larger number of women infected with HIV disease, the morbidity associated with anogenital warts may be significant.
Human Papillomavirus Infection in Children and Adolescents
HPV infection is highly prevalent in sexually active adolescent and young adult women (2), but only a small proportion of infections lead to condylomata acuminata (genital warts) or anogenital cancers. Rarely, children may develop condylomata acuminata (genital warts) through vertical transmission, sexual contact, or close contact with a caregiver (3,4). In adolescent and adult women, HPV infection may cause condylomata acuminata, as well as cervical, vaginal, and vulvar precancers and cancers. However, cervical carcinoma is extremely rare in the adolescent age group (5).
Cervical cytology testing, previously described as Papanicolaou (Pap) testing but now more frequently performed using liquid-based cytologic testing, is used to screen cervical epithelial cells for evidence of cervical intraepithelial neoplasia (CIN), also termed dysplasia, which is diagnosed histologically. Epithelial cell abnormalities found on cervical cytology that may indicate cervical dysplasia include atypical squamous cells of undetermined significance (ASC-US); atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H); low-grade squamous intraepithelial lesion (LSIL); and high-grade squamous intraepithelial lesion (HSIL) (6). Cervical dysplasia may be a precursor of carcinoma in situ and invasive cervical carcinoma. Cervical dysplasia is classified as mild (CIN 1), moderate (CIN 2), or severe (CIN 3, which includes carcinoma in situ).
Progression from infection with high-risk HPV types to precancerous CIN grades 2 and 3 may occur when the HPV infection persists over several years causing oncogenic changes. The time frame from HPV infection to CIN 2 or 3 usually takes several years, and the development from CIN 2 to 3 to cancer typically takes 10 to 20 years (7). Of note, by definition, low-risk HPV types are not associated with cervical cancer.
Recent research has provided new information about the epidemiology and natural history of HPV infection and abnormal cervical cytology (8,9,10), new techniques for cytologic screening and HPV testing (11,12,13), and strategies for cytologic screening and management of abnormal cytology (14). There are also two U.S. Food and Drug Administration (FDA)-approved vaccines, Gardasil and Cervarix, which protect against new infections with the two most aggressive high-risk types, HPV 16 and HPV 18. These types cause approximately 70% of cervical cancers. Gardasil also protects against two low-risk types, HPV 6 and 11, which cause >90% of genital warts. Because these vaccines do not protect against all of the high-risk HPV types, and because we do not know yet the duration of protection, it is recommended that cervical cytology screening be continued as per national guidelines (15,16). Moving forward, much research is being directed toward optimizing the combination of prophylactic vaccination and screening.
Biology of Human Papillomavirus Infection
Virology and Genetics
Human papillomaviruses are small, circular, double-stranded DNA viruses belonging to the papovavirus family. The virus infects and replicates in basal epithelial cells. The HPV genome is divided into two regions. The long control region (LCR) is noncoding and contains transcription enhancer genes and promoter elements (17,18). The coding region consists of an early and a late coding region. The early region contains six open reading frames that encode for the proteins E1, E2, E4, E5, E6, and E7; these control viral replication, transcription, and cellular transformation. In cancer-associated (high-risk) HPV types, the E6 and E7 proteins interfere with cell cycle control and result in uncontrolled cell proliferation by binding to and inactivating the tumor suppressor gene products p53 and retinoblastoma protein (19). The late region encodes for L1 and L2, which are structural proteins. They self-assemble into the viral capsid, which interacts with a receptor on the target cell, facilitating entry of viral DNA.
Human Papillomavirus Typing
Human papillomaviruses are epitheliotropic, and each type preferentially infects a specific anatomic site. They are divided into cutaneous and mucosal types; the mucosal types are found in the anogenital tract and aerodigestive tract. HPVs are genotyped on the basis of their genetic similarities; different types share <90% homology (20). Over 100 different genotypes have been sequenced and classified, at least 30 of which infect the genital tract (20). These genital types have been classified into low risk and high risk based on their association with condylomata and cervical cancer, respectively (Fig. 20-3). Although both types can regress spontaneously, persistent infection with low-risk types is associated with the development of genital warts (condylomata), and persistent HPV infection with high-risk types is associated with the development of cervical dysplasia and cervical cancer. When HPV infection occurs, the low-risk types remain extrachromosomal (episomal); in contrast, the genomes of high-risk types integrate into cellular host DNA in most human cervical carcinomas. High-risk HPV DNA, mitochondrial
RNA (mRNA), and proteins are found in the vast majority of cervical dysplastic lesions and carcinomas, and HPV is found in 99.7% of cervical cancer tissues (21,22); thus, high-risk HPV types are generally accepted as the etiologic agents of cervical cancer. Other anogenital cancers, oropharyngeal and tongue cancers, and a proportion of esophageal cancers have also been shown to be associated with high-risk HPV infection (23,24,25,26). While testing for high-risk HPV types in older women may be useful for determining risk of cancer, there is no added value to testing for either low-risk or high-risk HPV types in the adolescent patient, and HPV testing should not be used in this age group (16). If HPV testing is inadvertently performed, a positive result should not influence management (16).
RNA (mRNA), and proteins are found in the vast majority of cervical dysplastic lesions and carcinomas, and HPV is found in 99.7% of cervical cancer tissues (21,22); thus, high-risk HPV types are generally accepted as the etiologic agents of cervical cancer. Other anogenital cancers, oropharyngeal and tongue cancers, and a proportion of esophageal cancers have also been shown to be associated with high-risk HPV infection (23,24,25,26). While testing for high-risk HPV types in older women may be useful for determining risk of cancer, there is no added value to testing for either low-risk or high-risk HPV types in the adolescent patient, and HPV testing should not be used in this age group (16). If HPV testing is inadvertently performed, a positive result should not influence management (16).
Epidemiology and Natural History of Human Papillomavirus Infection
Human Papillomavirus in Children
HPV in children may be acquired perinatally or through sexual abuse (27,28,29). Other modes of transmission, including autoinoculation, heteroinoculation, and indirect transmission via fomites, remain possible but controversial (30). Studies have demonstrated the presence of asymptomatic HPV on the skin of 50% to 70% of normal healthy infants from 1 to 4 months of age (31), and HPV has been found within the oral cavities of infants without disease (32,33). Sequelae of vertically transmitted HPV infection in infants are uncommon, but include respiratory papillomas and vulvar and anal condylomata. Development of vulvar condylomata due to vertical HPV transmission is rare after age 3 and should raise concern for sexual abuse (27). The risk of developing subsequent cervical, vulvar, or vaginal intraepithelial neoplasia after perinatal acquisition or sexual abuse is not known, although high-risk types have been shown to persist in some children (34). However, HPV-related cancers are rarely reported in this age group (5).
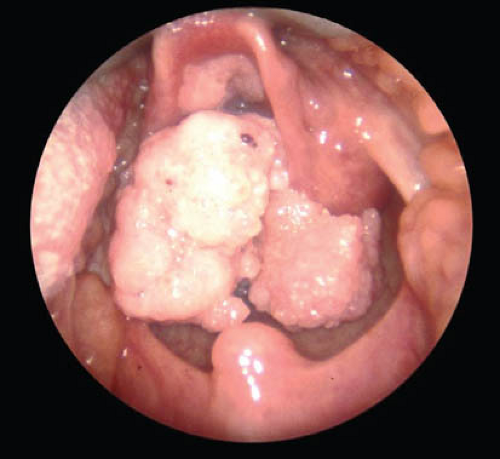
Recurrent respiratory papillomatosis (RRP) is a rare outcome of vertical transmission of HPV from mother to newborn, and is usually caused by HPV types 6 and 11 (35,36). Armstrong and colleagues estimated that there were 80 to 1500 incident cases and 700 to 3000 prevalent cases of RRP in the United States in 1999 (37). RRP is characterized by recurring papillomas in the upper respiratory tract, usually the larynx, and is most commonly diagnosed in children 2 to 3 years of age (Fig. 20-4). Although RRP is rare, it may be associated with high morbidity in some children (38).
Human Papillomavirus Infection in Adolescents
HPV infection is highly prevalent in the United States. Dunne and colleagues estimated that 24.5% of females ages 14 to 19 and 44.8% of females ages 20 to 24 were found to have polymerase chain reaction (PCR) evidence of HPV. However, the prevalence of HPV vaccine (types 6, 11, 16, and 18) was uncommon, detected in only 3.4% of female patients overall (2,38,39). Little was known about the natural history of HPV infection in adolescents until Moscicki and colleagues reported the results of a longitudinal cohort study of 618 HPV-positive young women 13 to 21 years of age (40). Participants were followed every 4 months with cytology, colposcopy, and HPV DNA testing. The investigators found that regression rates of HPV in these young women, defined as having at least three negative tests for HPV DNA by 30 months of follow-up, were high, particularly for those with low-risk HPV types. The regression rate for participants with low-risk HPV types was 90%, and for participants with high-risk HPV types was 75%. Of the young women who were HPV positive at baseline or during a follow-up visit, only 22% developed LSIL over a median follow-up period of approximately 60 months (41). Those who had high-risk HPV types were at increased risk for developing HSIL: the odds of developing HSIL for young women testing positive for high-risk HPV at more than or equal to three of four preceding visits were 14.1 compared to those who were negative for high-risk HPV (40). However, 88% of those subjects persistently positive for high-risk HPV for at least 1 year did not develop HSIL during the study period. The authors suggest that cofactors or more prolonged infection may be important in the development of HSIL. A recent study by Winer and associates of women ages 18 to 22 years demonstrated that the median time to HPV clearance (as defined by negative
testing) after HPV infection was 9.4 months and 90.6% of infections were undetectable within 2 years. However, 60% of patients were infected with multiple HPV types, and 19.4% of infections that became undetectable were redetected (9). A number of studies have similarly demonstrated that persistence of high-risk HPV infection is associated with development of cervical dysplasia (42,43,44,45,46).
testing) after HPV infection was 9.4 months and 90.6% of infections were undetectable within 2 years. However, 60% of patients were infected with multiple HPV types, and 19.4% of infections that became undetectable were redetected (9). A number of studies have similarly demonstrated that persistence of high-risk HPV infection is associated with development of cervical dysplasia (42,43,44,45,46).
Condylomata Acuminata
Condylomata acuminata, or genital warts, are a common clinical manifestation of genital HPV infection. The prevalence rate of symptomatic external genital warts in the general population is approximately 1.2 per 1000 females overall, with the highest rates in women ages 16 to 20 (4.6/1000), though rates are likely to be higher in young women because HPV prevalence declines with age (47,48). Genital warts may resolve spontaneously without treatment, likely due to acquired cellular immune responses (49,50,51); some investigators report up to a 40% spontaneous resolution rate in those participating in treatment trials for genital warts who were treated with placebo agents. A higher wart burden at presentation is associated with longer time to clearance (52).
Abnormal Cytology
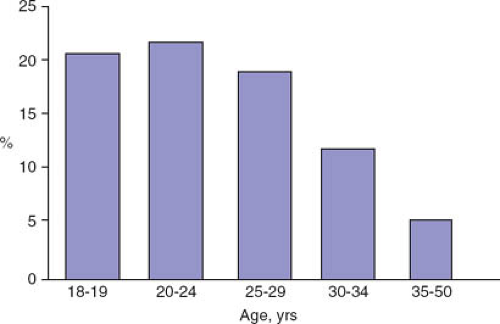
The Pap test and other cytologic testing methods are used to screen the cervix for abnormal cells suggestive of dysplasia, which is then diagnosed by colposcopically directed biopsies. In adult women, the prevalence rates of ASC-US and LSIL mirror rates of HPV infection; rates are highest in 20- to 24-year-old women and decline with age (Fig. 20-5) (47). The prevalence of HSIL cytology rises slightly in 20- to 24-year-olds compared with 18- to 19-year-olds, but then remains fairly steady until age 35, when rates decline again. In contrast, the prevalence of biopsy-proven CIN 3 or more severe abnormalities rises with age: this is consistent with the natural history of CIN.
Although abnormal cytology in adolescents is common, few have HSIL. Sadeghi and colleagues reviewed almost 200,000 Pap tests in the United States in the early 1980s. The investigators reported that 2% of smears were positive for CIN 1 or 2 and 0.1% were positive for CIN 3 (note: this study uses prior cytologic nomenclature) (53). More recent studies involve fewer participants but demonstrate higher rates of abnormal cytology. Rates of ASC-US range from 4% to 13%, LSIL from 3% to 9%, and HSIL from 1% to 3% (54,55,56,57).
In one study of the natural history of LSIL in adolescents, Moscicki and colleagues reported that of all participants with LSIL in a longitudinal study, the regression rate after 3 years was very high—95% (58). This finding is in contrast to adult women, in whom only 50% to 80% of LSIL will regress. In the same study sample, the regression rate of LSIL in those with high-risk HPV was 80%, while only 6% progressed. The future risk of developing HSIL in adolescents with normal cytology at baseline was also low, even in those with high-risk HPV. Finally, the progression of SIL generally takes several years and cervical carcinoma is exceedingly rare in adolescents. Surveillance, Epidemiology, and End Results (SEER) data from 2003 to 2007 demonstrated that the incidence of cervical cancer was 0.14 per 100,000 women in the 15- to 19-year-old age group, and only 1.6 per 100,000 women in the 20- to 24-year-old age group (5).
Moreover, further analysis of the cancers in these age groups shows them to be primarily sarcomas and adenocarcinomas, which are not easily detected through screening. The December 2009 American College of Obstetricians and Gynecologists (ACOG) recommendations to start cervical cytology screening at age 21 regardless of the age of onset of sexual intercourse are based in part on the rarity of cervical cancer in younger women (16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree