Human Immunodeficiency Virus Type 1 Infection
Michael T. Brady
 EPIDEMIOLOGY
EPIDEMIOLOGY
Worldwide, it is estimated that 2.5 million children younger than 15 years are infected with human immunodeficiency virus type 1 (HIV-1), with more than 2.2 million HIV-1-infected children in Africa alone.1 In the United States, rates of new pediatric HIV infections increased from 1982 until 1995. Since 1995, the use of antiretroviral agents to prevent mother-to-child-transmission of HIV-1 has significantly reduced perinatal transmission. Rates of new HIV-1 infections in children younger than 15 years in the United States have declined from a high of 2500 per year in the early 1990s to approximately 100 to 200 per year.2
More than 95% of HIV-1–infected infants and children acquire their infection vertically, during gestation, especially later gestation, or during labor and delivery. An increasing proportion of women with HIV-1 contracted their infection through heterosexual contact, although injection drug use and substance abuse still play a significant role.2 Ethnic minorities and individuals with low income are markedly overrepresented among HIV-1-infected women and HIV-1–infected infants and children in the United States.2 Adolescents participating in adult risk behaviors (sexual activity and injection drug use) are increasing and represent the largest number of new pediatric infections.2
The timing and mechanism of perinatal HIV-1 transmission are not completely understood. It appears likely that in the preantiretroviral era, the majority of infants were infected in the peripartum period, either through transplacental passage of virus (late pregnancy or at the time of labor) or by exposure to HIV-1 during birth. Because antiretroviral therapy has been successful in reducing perinatal HIV-1 transmission, particularly late in pregnancy and at the time of delivery, transmission earlier in pregnancy now represents the more common period of transmission. Postnatal transmission through breast-feeding is well documented,3 and this fact underlies the recommendation that mothers with HIV-1 infection should not breast-feed if safe, alternative infant nutrition is available. In areas where a safe alternative is not available, breast-feeding exclusively should be maintained until the infant can obtain adequate nutrition without breast-feeding.
Of children born to HIV-1–infected women not receiving antiretroviral treatment, 13% to 40% will be infected.5 Many maternal and obstetric factors that contribute to the risk of perinatal transmission have been identified. Prematurity, advanced maternal disease, exposure to maternal blood, and higher maternal viral load are associated with higher risks for HIV transmission.5-7 Of all identified risk factors, it appears that reducing the mother’s viral load offers the greatest opportunity to reduce perinatal transmission.8 Mode of delivery (cesarean delivery appears to confer some protection from perinatal transmission of HIV-1) may also reduce the risk of perinatal transmission.9
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
HIV-1 preferentially infects lymphocytes with the CD4 surface antigen, which, together with the cytokine cell receptors CCR5 and CXCR4, acts as the viral receptor. This lymphocyte subset, which includes helper lymphocytes with critical roles in maintaining immune responsiveness, also shows gradual attrition with progression of disease. 
HIV-1 does infect cell types other than lymphocytes. HIV-1 infection of monocytes, unlike that of CD4+ lymphocytes, may not lead to death of the cell. Infected monocytes may act as latent but inducible reservoirs of virus and may carry virus to organs, particularly the brain, in which they become resident. Hybridization experiments demonstrate HIV-1 viral nucleic acid in chromaffin cells of the intestinal mucosa, glomerular and tubular epithelia, and astroglia. HIV-1–related pathology involves many organs, although it is often difficult to know whether injury is primarily a consequence of local virus infection, immunemediated cytotoxic effects, or other associated infectious complications.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Childhood and Adult HIV-1 Infection
The hallmark stages of HIV-1 infection following acquisition in child- or adulthood are an acute infection phase (seroconversion syndrome), often with flulike symptoms, accompanied by high-grade viremia; followed by a period of immune containment of viral replication, during which the individual is usually free of symptoms; and a final period of progressive symptomatic immune compromise, with increasing viral replication. During the second asymptomatic phase, gradual and progressive abnormalities of immune function are apparent on testing. Viral load (HIV RNA by polymerase chain reaction [PCR] quantification of viral particles in the plasma, representative of viral replication rate) is variable, but is usually lower than viral load levels detected during the acute infection phase, and generally remains stable for months to years. The rapidity with which infected adults and children progress through the second (clinically stable) phase can be predicted to some degree by determining the individuals’ CD4+ cell count and viral load.10,11 Lower CD4+ cell counts and higher viral loads are each independent predictors of more rapid disease progression.10,11 The final phase, with symptomatic immune compromise, end-organ dys-function, and HIV-1–associated malignancies, is correlated with increasing viral replication and often a change in viral type (nonsyncytial → syncytial), viral tropism (preferential use of cytokine receptor: CCR5 → CXCR4), profound attrition of CD4+ lymphocytes, and severe immune dysregulation (not just immune deficiency) and opportunistic infections.
Infant HIV-1 Infection
Perinatal HIV-1 infection is generally clinically silent at birth, although the “incubation period,” or interval before symptoms of HIV-1 infection become manifest, is generally shorter following perinatal infection than in adult HIV-1 infection. In a few instances, adenopathy may be detected in the first month of life.
Clinically silent abnormalities of immune function often precede HIV-1–related symptoms, although the immunologic assessment of infants at risk is complicated by several unique factors (see Diagnosis). During this phase, immune dysregulation is often apparent on testing, especially with regard to B-cell percentage and function; hypergammaglobulinemia with production of nonfunctional antibodies (polyclonal B-cell stimulation) is more common among HIV-1–infected children than among adults, typically noted as early as 3 to 6 months of age.12,13 Despite the abundance of immunoglobulins, there is an inability to respond to new antigens with appropriate immunoglobulin production.12,13 This critically affects infants without prior antigen exposure, contributing to the greater frequency and severity of invasive bacterial infections seen in pediatric HIV-1 infection. Infants and young children have much higher absolute numbers of lymphocytes, including CD4+ lymphocytes.14 Depletion of CD4+ lymphocytes may not be as readily apparent in HIV-1–infected infants and young children, and the absolute CD4+ lymphocyte count may not be as predictive of the risk for opportunistic infections.
The earliest and most common HIV-1–associated symptoms in infancy are nonspecific and rarely diagnostic. As listed by the Centers for Disease Control and Prevention in the 1994 Revised Classification System (Table 315-1),15 the first abnormalities detected include fever, failure to thrive, hepatomegaly and splenomegaly, generalized lymphadenopathy, parotitis, and diarrhea. Prior to the early use of aggressive combination antiretroviral therapy, approximately 90% of perinatally HIV-1–infected infants would manifest one or more of these symptoms in the first year of life. The conditions that best discriminate between untreated uninfected infants and HIV-1–infected are chronic candidiasis, parotitis, persistent lymphadenopathy, and hepatosplenomegaly.16 Otitis media, rhinitis, unexplained fever, and chronic diarrhea were not significantly more common in HIV-1–infected infants than in uninfected infants.
Approximately 20% of HIV-1–infected infants present with rapidly progressive immune compromise and/or an AIDS-defining condition such as Pneumocystis jirovecii pneumonia (PCP), or serious bacterial or fungal infections within the first 3 to 6 months of life.
HIV-1–infected infants, including some receiving antiretroviral therapy, may have some degree of growth failure, recurrent or chronic fever, developmental delay, persistent adenopathy, or hepatosplenomegaly. With the exception of linear growth abnormalities, most of these symptoms are significantly less common and/or less severe with aggressive combination antiretroviral therapy. With successful antiretroviral therapy (good compliance and undetectable viral load), opportunistic infections are extremely rare. The development or worsening of HIV-1–related symptoms while receiving effective antiretroviral therapy suggests clinical failure and possible resistance to one or more of the medications in the treatment regimen.
 SPECIFIC ASSOCIATED DISORDERS
SPECIFIC ASSOCIATED DISORDERS
Pneumocystis jirovecii Pneumonia
Pneumocystis jirovecii pneumonia (PCP) is the most common of the AIDS indicator diseases in children and adults, and it previously affected approximately one third of HIV-1–infected infants and children.18 The median age for presentation following perinatal HIV-1 infection is approximately 9 months of age, although there is a peak at 3 to 6 months of age among rapidly progressing and previously unidentified HIV-1–infected infants. This infection is usually a primary infection in HIV-1–infected children, presenting subacutely or abruptly with fever, cough, tachypnea, and rales. PCP may be difficult to distinguish clinically and radiologically from other pulmonary infections at this age. Because intravenous trimethoprim-sulfamethoxazole (TMPSMX) and corticosteroids (for those infants with significant oxygen requirement) given early in the course may lead to marked improvement, diagnostic bronchoalveolar lavage should be considered early in the course of illness in the infant with a consistent clinical presentation and risk factors for HIV-1 infection. In very young infants, PCP may still be associated with a high mortality rate. Milder disease may occur in both young infants and older children, and permits long-term survival.
Lymphoid Interstitial Pneumonitis
This chronic interstitial infiltration of the lungs has been described in a small number of HIV-infected adults, but is seen in 20% to 25% of HIV-infected children. There may be an association with Epstein-Barr virus infection.19 The condition is characterized by a chronic course with intermittent exacerbations (often during intercurrent respiratory infections). Chronic chest infiltrates seen on x-ray often suggest the diagnosis, but only open-lung biopsy is definitive. Hypoxia is seldom severe until the condition has been present for many years; improvement with corticosteroid use has been reported. As a presenting symptom of HIV-1 infection, lymphoid interstitial pneumonitis (LIP) appears to be associated with a better prognosis than other AIDS indicator diseases, and is often seen in a symptom cluster with marked hyper-gammaglobulinemia, parotitis, and massive adenopathy.
Table 315-1. 1994 Revised HIV Pediatric Classification System: Clinical Categories
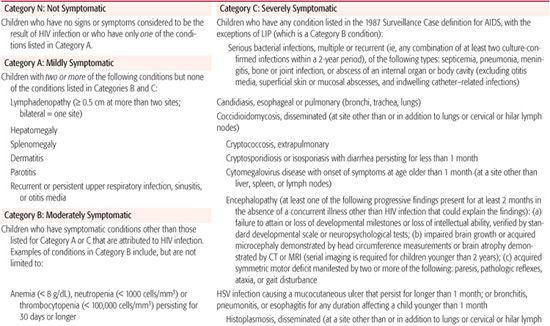

Infection
Recurrent Bacterial Infections These are defined as two or more episodes of sepsis, meningitis, pneumonia, internal abscesses, or bone and joint infection. The frequency of bacterial infections is far less in children receiving appropriate antiretroviral therapy, PCP prophylaxis and pneumococcal conjugate vaccine. Less invasive bacterial infections, such as chronic or recurrent sinus infections, otitis media, and pyodermas, are somewhat more common in HIV-1–infected children. Streptococcus pneumoniae is the most frequent blood isolate in HIV-1–infected children, although gram-negative enterics (particularly Salmonella), staphylococcal, and even pseudomonal bacteremia are seen more commonly in HIV-1–infected children.
Opportunistic Infections More than a dozen specific opportunistic infections meet the AIDS definition (Table 315-1).15 After Pneumocystis jiroveci pneumonia (PCP), the most common opportunistic infection in pediatric AIDS patients are Candida esophagitis and Mycobacterium avium complex infection. The most common infections caused by viruses are recurrent, prolonged, or disseminated infections with the cytomegalovirus (CMV), particularly of the gastrointestinal tract (CMV retinitis occurs in children but is less common than in adults), and recurrent, extensive, and atypical infections with herpes simplex and varicella-zoster. Despite the long list of pathogens causing unusually severe or protracted illness in HIV-1–infected children, common respiratory viruses, including respiratory syncytial virus, seldom cause complicated illness.
Progressive Neurologic Disease
Central and peripheral nervous system disease occurs more commonly and at an earlier point in HIV-1 disease in children than it does in adults.20 Prior to availability of antiretroviral therapy, as many as 25% of HIV-infected infants had central nervous system (CNS) involvement manifested as a static encephalopathy, usually presenting as developmental delay in the first year of life.20 Neuroimaging may reveal cerebral atrophy, white matter abnormalities, and/or basal ganglion calcifications, although the severity of imaging abnormalities often does not correlate with clinical findings. Because this rapidly progressive syndrome is identified less commonly in the era of combination antiretroviral therapy, it would appear that successful control of viral replication can either prohibit ongoing CNS damage from virus already present in the CNS or reduce new virus entry into the CNS.
Growth
Chronic failure to thrive was seen in many infants and children with advanced HIV-1 infection, and it is nearly always multifactorial. Anorexia can result from generalized malaise and fatigue, as well as from mouth sores associated with thrush (Candida albicans and other yeasts), herpes simplex, and aphthous ulcers. Central nervous system deficits, including lethargy, weakness in swallowing, neuroendocrine abnormalities, malabsorption and diarrhea resulting from numerous enteric pathogens as well as primary HIV-1 infection, and infection-induced catabolism, frequently contribute to this vexing problem. More recent combination anti-retroviral therapies have resulted in better maintenance of weight gain, or in diminished weight loss. However, linear growth has not improved as dramatically (possibly because of resistance to growth hormone). In addition, combination therapies with and without protease inhibitors are associated with marked elevation of triglycerides and cholesterol and changes in fat deposition (lipodystrophy syndromes—increased fat deposition in the abdomen and upper back [lipohypertrophy] with loss of fat from the face and extremities [lipoatrophy]).21
Other Organ Involvement
Hepatic involvement in pediatric HIV-1 infection often takes the form of hepatomegaly with mild-to-moderate, fluctuating transaminitis. Less common is a severe cholestatic hepatitis seen in infected infants in the first year of life, with a poor prognosis.22-27 Liver abnormalities may be exacerbated by concomitant infection with the common viruses causing hepatitis (CMV; hepatitis A, B, and C; and the Epstein-Barr virus), by HIV-1 infection itself, or by many of the medications used to treat HIV-1 and its infectious complications. Renal disease is not uncommon, with proteinuria the most likely finding.23 Focal glomerulosclerosis and mesangial changes have been identified in children with advanced HIV-1 infection. Prior to antiretroviral therapy, cardiac abnormalities had been demonstrable in as many as 50% of children at all stages of HIV-1 disease, although the incidence of symptomatic cardiomyopathy is only 12% to 20% and it occurs late in advanced disease; ventricular dysfunction and pericardial effusion are the most commonly encountered echocardiographic abnormalities.24 Autoimmune phenomena include Coombs-positive hemolytic anemia, thrombocytopenia, and aphthous ulcers. Kaposi sarcoma and other secondary cancers occur but are uncommon in HIV-1–infected children.
 DIAGNOSIS
DIAGNOSIS
Early diagnosis of the infected infant is desirable, but early recognition of the infant at risk for HIV-1 infection may be equally as important. Only if HIV-1 infection in the pregnant woman is identified will there be an opportunity to intervene during pregnancy with both antiretroviral therapy and necessary psychosocial services. Thus, HIV-1 antibody testing and counseling should be a routine part of pregnancy care.17 Initial testing of the mother should be performed in the first trimester (or first visit if later than first trimester). Repeat HIV antibody testing in the third trimester (prior to 36 weeks’ gestation) should be considered for pregnant women perceived to be at increased risk for HIV-1 infection.17
The persistence of transplacentally acquired antibody to HIV-1 in the infant complicates the use of conventional IgG antibody tests (enzyme-linked immunosorbent assay [ELISA] and/or Western blot) in diagnosing HIV-1 infection in infancy. Because such HIV-1 antibodies may remain in uninfected infants’ blood for up to 18 months, diagnosis of HIV-1 infection in the infant at risk requires the culture of virus from the infant (HIV-1 culture) or the demonstration of viral nucleic acids (HIV-1 DNA or HIV-1 RNA detection by polymerase chain reaction [PCR]) or HIV antigen (p24 antigen).25,26 With greater than 99% confidence, virologic testing with either HIV-1 DNA PCR or HIV-1 culture of peripheral blood can be expected to establish or exclude the diagnosis of HIV infection in an infant by 4 months of age.25,26 Performed appropriately, these tests have an acceptably low rate of false positivity and can be relied on to confirm infection at any age. The sensitivity of each is somewhat lower in the immediate perinatal period (prior to 4 weeks of age), making serial testing necessary. For prospective monitoring of infants at risk, diagnostic virologic testing is recommended at least three to four times during the first 4 months after birth (last test at or after 4 months of age).25,26
The optimal schedule for HIV antibody testing of the HIV-exposed infant would include birth, 14 to 21 days, 4 to 6 weeks, and 4 to 6 months of age. The birth test is obtained to identify infants infected at birth. Because zidovudine monotherapy is used commonly in HIV-1–exposed newborns, this early test can identify infection and avoid continued mono-therapy with risks for development of resistance. Presumptive noninfection with HIV-1 can be determined with negative tests at ≥ 2 and ≥ 4 weeks of age (or one negative test at ≥ 8 weeks of age).25 Definitive exclusion of infection with HIV-1 requires two negative virologic tests with one at ≥ 4 weeks of age and one at ≥ 4 months of age.26 Most experts are still recommending that HIV-1 antibody testing be repeated at 18 months of age to ensure noninfection of the child.
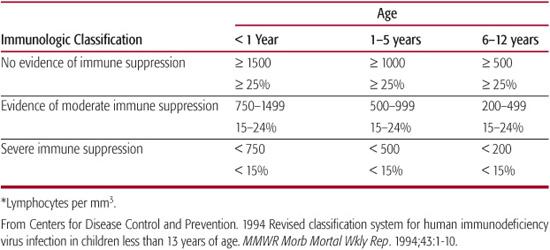
When infants or children without recognized risk factors for HIV-1 infection present with findings or signs compatible with immunodeficiency, the diagnosis of HIV-1 should be entertained along with other causes of immunodeficiency. Infants and children with HIV infection often have normal numbers of total lymphocytes, and as many as 15% of patients with pediatric AIDS may have a normal absolute number of CD4+ lymphocytes. However, the CD4+ cell percentage is relatively similar at all ages and may detect CD4+ lymphocyte abnormalities that are the result of HIV-1 infection in some infants and young children with normal absolute CD4+ lymphocyte counts.14 However, in infants younger than 1 year, neither absolute numbers nor percentage of CD4+ lymphocytes can be relied upon entirely to identify HIV-1–infected infants with immune dysfunction and an associated risk for opportunistic infections. Age-specific normal parameters for CD4+ lymphocyte count and CD4/CD8 ratio show higher absolute CD4+ numbers and wider ranges in early infancy, followed by a gradual decline over the first several years (Table 315-2).15 It is thus imperative to refer to age-adjusted standards for CD4+ cell counts. These are ideally established from observation of uninfected infants born to infected mothers.
In children from 18 months of age through adolescence, a confirmed positive serologic test for antibody to HIV-1 (positive ELISA confirmed by Western blot or other confirmatory test) is usually sufficient to establish the diagnosis of HIV-1 infection.
Table 315-2. Age-Specific CD4+ T-Lymphocyte Count and Percentage of Total Lymphocytes* for Children Younger Than 13 Years
 MANAGEMENT
MANAGEMENT
Care of children with or at risk of HIV-1 infection requires a skilled team approach that includes medical specialists, primary care physicians, nurses, social workers, nutritionists, pharmacists, and developmental experts. Extensive psychosocial support is often necessary for families.
After perinatal HIV-1 infection or perinatal exposure is diagnosed in an infant or child, testing may be required for siblings and on parents and their sexual partners. The initial visit of the HIV-1–infected or perinatally exposed child should include a thorough physical examination and laboratory evaluation as a baseline for further monitoring. This and subsequent examinations should devote particular attention to rate of growth, pulmonary symptoms and findings, location and size of enlarged lymph nodes, liver and spleen size, and neurologic and developmental assessment.
Medical care of infants at risk of HIV-1 infection whose infection status remains uncertain requires careful prospective evaluation for early signs and symptoms of HIV-1 infection. Because serious bacterial infection or opportunistic infections such as Pneumocystis jiroveci pneumonia (PCP) may be the first clinical presentation associated with their HIV-1 infection; febrile episodes and respiratory illnesses should be aggressively managed in these infants. Immunologic testing (complete blood counts, CD4+ cell count, and quantitative immunoglobulins) will aid in decisions regarding PCP prophylaxis, intravenous immunoglobin (IVIG) therapy, and antiretroviral therapy, and should be performed every 3 to 4 months.28 Quantitative HIV-1 RNA by polymerase chain reaction (PCR) (viral load) and CD4+ cell counts are independent predictors for risk of disease progression. Clinical well-being in HIV-1–infected children can be estimated by assessment of rate of growth (weight, length, and head circumference growth velocities), developmental achievement, and experience with bacterial and viral infections. Following these clinical and laboratory parameters should assist in decisions concerning initiation and switching combination antiretroviral therapies, prophylaxis for opportunistic infection, nutritional interventions, and psychosocial support efforts.
Pneumocystis jirovecii Pneumonia Prophylaxis
To reduce the risk of PCP, HIV-1–exposed infants should begin PCP prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) at 4 to 6 weeks of age unless they have been determined to be presumptively noninfected with HIV.25-27 They should continue PCP prophylaxis until infection status is established. In uninfected infants, PCP prophylaxis can be discontinued. If HIV-1 infection is proven, PCP prophylaxis must be continued through the first year of life, and longer if the child’s immunologic status warrants it.27 (evidence of severe immune suppression; Table 315-1). TMP-SMX (150 mg TMP/m2/day in 2 divided doses, orally, 3 consecutive days per week) is the drug of choice for prophylaxis of PCP, and this may also provide some protection from serious bacterial infections. Alternatives to TMP-SMX for patients who cannot tolerate this combination include dapsone, atovaquone, and pentamidine (aerosol).27
Antiretroviral Therapy
Updated information on the most current recommendations for treating HIV-infected children can be obtained by accessing the Web site of the HIV/AIDS Treatment Information Service (www.aidsinfo.nih.gov/).
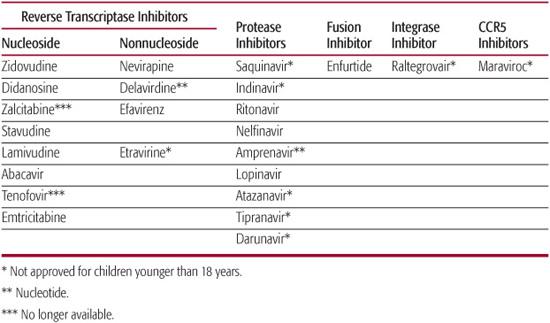
Antiretroviral therapy for HIV-1–infected children has undergone a dramatic evolution over the past decade. The currently available antiretroviral agents can be divided into six distinct categories: (a) nucleoside/tide reverse transcriptase inhibitors (NRTI), (b) nonnucleoside reverse transcriptase inhibitors (NNRTIs), (c) protease inhibitors (PIs), (d) fusion inhibitors, (e) cytokine receptor inhibitors, and (f) integrase inhibitors (Table 315-3). The most effective combinations have included two nucleoside reverse transcriptase inhibitors in combination with either a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor.28 These combinations are known as HAART, which stands for highly active antiretroviral therapy. These three-drug regimens have been associated with dramatic reductions in viral load (approximately 40–60% of children who adhere to these regimens reach undetectable levels of virus in their plasma [less than 50 copies/mL]) and improvements in, or preservation of, CD4+ cell counts and lymphocytic function.28
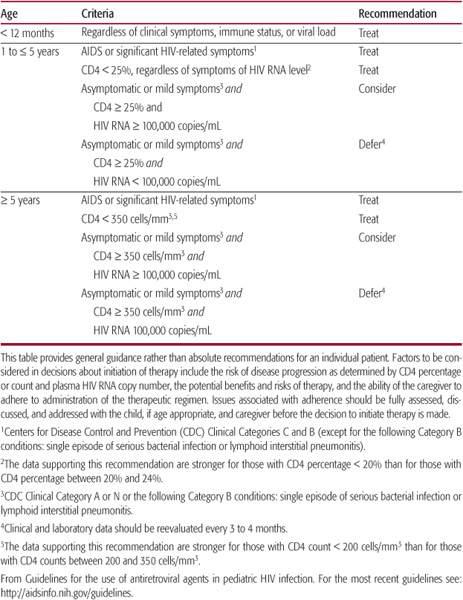
Because approximately one sixth of HIV-infected children experience rapid progression beginning in the first year of life, HIV clinicians start aggressive combination antiretroviral therapy in every child younger than 1 year as soon as the diagnosis is established.28 For children whose HIV-1 infection status is not determined until after the first year of life, it may be reasonable to defer initiation of treatment until there is clinical, immunologic, or virologic evidence that disease progression is imminent28 (Table 315-4). However, once a decision is made to treat, it should be expected that anti-retroviral therapy will continue for the remainder of the child’s life. Antiretroviral regimens that do not optimally suppress viral replication are associated with poorer clinical outcomes and a higher likelihood for the development of resistance to the antiretroviral medications being administered. Compliance is a significant problem in both HIV-1–infected children and adults. Successful aggressive antiretroviral regimens require the administration of multiple medications with multiple doses per day. Psychosocial support of the family and careful coaching in how best to administer medications may enhance adherence with these cumbersome combination regimens.
Decisions concerning change in antiretroviral therapy should be guided primarily by the child’s prior medication history and HIV-1 genotype at time of treatment failure. A single agent should never be added to failing therapy. The optimal approach to changing therapy is to make a complete shift in prescribed medications, with the hope that the new regimen includes at least two medications to which the child’s virus is susceptible and, optimally, to which the child has not been previously exposed. If the child has already received a protease inhibitor, it may be appropriate to replace the protease inhibitor with a nonnucleoside reverse transcriptase inhibitor, or vice versa. Alternate categories of antiretroviral therapy are now available (eg, fusion inhibitors, integrase inhibitors, cytokine receptor agonists) and may be sufficiently different from those previously administered to make cross-resistance less likely.
Intravenous Immunoglobulins
For patients receiving combination antiretroviral therapy and trimethoprim-sulfamethoxazole (TMP-SMX) for Pneumocystis jiroveci pneumonia (PCP) prophylaxis, the benefit of intravenous immunoglobin (IVIG) is diminished and, in most cases, not cost effective. For this reason, only HIV-1–infected patients with documented defects in the production of functional antibody or a history of recurrent bacterial infections while receiving antiretroviral therapy and PCP prophylaxis should be considered for prophylaxis with IVIG.
Table 315-3. Antiretroviral Agents
Immunization
Immunization schedules for HIV-1–infected and HIV-1–exposed children should include all vaccines given routinely to children of similar age with some exceptions for live virus vaccines.28-32 Varicella vaccine may be administered to asymptomatic or mildly symptomatic HIV-1–infected children with no evidence of immune suppression (Immunologic Category 1). Measles-mumps-rubella (MMR) vaccine can be given to HIV-1–infected children without evidence of severe immune suppression (Immunologic Categories 1 and 2). Rotavirus vaccine may be given to HIV-1–exposed infants and HIV-1–infected infants.
 PROGNOSIS
PROGNOSIS
Clinical status at the time of initial presentation appears to correlate with prognosis in perinatally infected children. Approximately 15% to 20% of HIV-1–infected infants have an early onset of disease symptoms (average age 4 months) and progress to AIDS within 1 year if not treated appropriately. The majority of HIV-1–infected infants, however, have longer asymptomatic periods, averaging approximately 5 years, with approximately 8% progressing to AIDS-defining illnesses per year of life. Prior to availability of antiretroviral regimens using three or more drugs, the estimated mean survival for children perinatally infected, but not symptomatic in the first year of life, was approximately 9.4 years. Since the availability of protease inhibitors or nonnucleoside reverse transcriptase inhibitors and optimal opportunistic infection prophylaxis, most pediatric HIV-1 treatment centers have documented marked reductions in both new opportunistic infections and mortality in HIV-1–infected children who experience immunologic improvement or stabilization following combination therapy. With appropriate anti-retroviral regimens and strict adherence to administering the prescribed medications, survival well into adulthood should be expected for the majority of HIV-1–infected infants, children, and adolescents.
Persons infected with HIV-1 during adolescence are often not diagnosed until early adulthood. Many at-risk adolescents fail to access systems likely to provide health care, counseling, or testing.
Natural history studies in adolescents are incomplete, although HIV infection in adolescents appears to resemble the disease time course, complications, and prognosis in adults.
 PREVENTION
PREVENTION
A solution to the worldwide HIV-1 pandemic would be the development of a safe and effective vaccine. Although many candidate vaccines are in various stages of investigation, there are still considerable obstacles impeding HIV-1 vaccine development. Prevention of HIV-1 infection in children entails the prevention of mother-to-infant transmission (perinatal) and prevention of transmission in adolescents who participate in adult risk behaviors. In the United States, there has been a marked reduction in perinatal HIV-1 transmission. This reduction has been accomplished primarily by first-trimester screening of pregnant women and the successful administration of antiretroviral therapy to pregnant HIV-1–infected women. Zidovudine starting at 14 to 32 weeks’ gestation reduces perinatal transmission from 25% to 8%.29 HIV-negative woman who are at high risk for acquiring HIV-1 infection (eg, infection drug users, partners are HIV-1–infected or injected drug users, women who exchange sex for money or drugs, women who have had a new sex partner or more than one sex partner during this pregnancy, women with signs consistent with the acute retroviral syndrome, women who receive health care in jurisdictions with elevated incidence of HIV-1 or AIDS among women 14–24 years of age) should have repeat testing in the third trimester, preferably prior to 36 weeks’ gestation.17 Many centers with the experience of managing HIV-1 infection during pregnancy have seen their rates of perinatal transmission fall to somewhere between 1% and 3%.30 This continued reduction in perinatal HIV-1 transmission has occurred because of more aggressive antiretroviral therapy and monitoring of viral loads in HIV-1–infected women during their pregnancies.
Table 315-4. Indications for Initiation of Antiretroviral Therapy in Children Infected with HIV
Administration of antiretroviral agents with minimal safety data for use during pregnancy has been fairly liberal because of the obvious benefits associated with appropriate treatment of the mother’s HIV-1 infection and the reduction in perinatal transmission. It will be important to monitor both short- and long-term outcomes of infants exposed perinatally to these therapies in order to determine the impact of antiretroviral therapy during gestation. 
Numerous studies have investigated the impact of the mode of delivery on the rate of perinatal transmission.32,33 Cesarean delivery does appear to provide some additional protection to the infant born to the HIV-1–infected woman.32,33 However, in the era of highly active antiretroviral therapy and the ability to monitor viral load closely, both the risks and the benefits of cesarean delivery must be clearly discussed with prospective parents before any change in the mode of delivery is considered. If the mothers viral load (HIV-1 RNA by polymerase chain reaction [PCR]) is consistently less than 1000 copies per mL, the mother may be counseled that additional benefit by cesarean delivery would be minimal.34 However, for others whose viral load is > 1000, they should be counseled that the benefits of cesarean delivery outweigh the risks.34
Breast-feeding is associated with HIV-1 transmission. For that reason, in areas where safe alternatives to breast milk are available, breast-feeding should be prohibited. Unfortunately, in many areas of the world where HIV-1 is particularly prevalent (eg, sub-Saharan Africa), safe alternatives to breast-feeding are not readily available. In these areas, breast-feeding may still be essential, but should be discontinued as quickly as possible because the duration of breast-feeding appears to have implications for increasing the rate of HIV-1 transmission. Also, when breast-feeding does occur, it should be the only source of milk for the infant. Infants receiving both breast milk and infant milk formula have a higher rate of HIV-1 infection than infants who are receiving breast milk only.36 If prenatal testing of the mother was not performed, all infants should be tested for HIV-1 antibody using a rapid test in the immediate postpartum period. If an infant is born to a mother who is at risk for HIV-1 infection as outlined previously and whose mother was not tested in the third trimester, the infant should be tested for HIV-1 antibody using a rapid HIV test as soon as possible after birth.
Adolescents who participate in behaviors identified with transmission in adults (eg, sexual promiscuity, needle sharing for the injection of illicit drugs or anabolic steroids) represent a growing population of HIV-infected children. Altering behavior is difficult in this population. Outreach education, particularly through the use of peer counselors, has been somewhat successful in taking the message to at-risk youth.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


