Chapter 45
Human Immunodeficiency Virus
David J. Wlody MD
Chapter Outline
In 1981, a cluster of cases of an unusual disorder, Pneumocystis carinii pneumonia (PCP), in five otherwise healthy men, initiated a search that culminated in the characterization of a new disease, acquired immunodeficiency syndrome (AIDS), and the identification of its causative agent, the human immunodeficiency virus (HIV). Subsequently, there has been an explosion of this disease in the United States. Geographically, a disease that once was limited to two or three urban areas is now found throughout the country. Further, the number of cases of HIV infection has reached epidemic levels. At the end of 2008, an estimated 1,178,000 persons were living with HIV in the United States, including more than 236,000 whose infection was undiagnosed.1 As of December 2011, more than 1,155,000 cases of AIDS had been reported to the U.S. Centers for Disease Control and Prevention (CDC). Some 636,000 people were reported to have died of AIDS and its complications by December 2010.2 HIV infection has exploded demographically from its initial isolation among homosexual men to its current endemic status among intravenous drug users, their sexual partners, and children born to women infected with HIV.
The impact of HIV infection in the developing world has been nothing less than catastrophic. As of 2012, the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimated that approximately 34 million people worldwide were infected with HIV (Figures 45-1 and 45-2). More than two thirds of these infections have occurred in sub-Saharan Africa, where nearly 5% of adults are infected with HIV and where 70% of all new infections that occurred worldwide during 2011 originated. It is encouraging that the number of new cases of HIV infection in sub-Saharan Africa has decreased by 25% between 2001 and 2011. Unfortunately, in at least nine countries, the number of new infections has increased by more than 25% in the same period.3
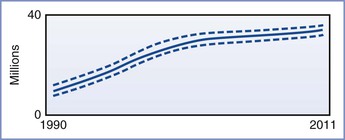
FIGURE 45-1 Estimated number of people living with HIV globally, 1990-2011. The bold line represents the estimate, and the dotted lines represent the high and low estimates. (From Global Report. UNAIDS report on the global AIDS epidemic 2012. Joint United Nations Programme on HIV/AIDS (UNAIDS), 2012. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf.)
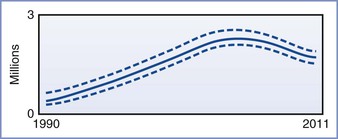
FIGURE 45-2 Adult and child deaths due to AIDS globally, 1990-2011. The bold line represents the estimate, and the dotted lines represent the high and low estimates. (From Global Report. UNAIDS Report on the global AIDS epidemic 2012. Joint United Nations Programme on HIV/AIDS (UNAIDS), 2012. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf.)
In the United States, although the rate of new infection in women appears to be decreasing, 21% of all new diagnoses of HIV infection identified in 2008 through 2011 occurred in women.2 Minority populations are disproportionately affected; 47% and 20% of all newly diagnosed infections in 2011 were seen in African-American and Hispanic populations, respectively.2 In 2009, HIV was the third leading cause of death in African-American women aged 35 to 44 years.4 Clearly, anyone providing anesthesia to pregnant women in the United States in the 21st century will care for patients who are infected with HIV. Neither medicolegal concerns nor fear of infection with HIV should prevent anesthesia providers from providing effective intrapartum analgesia and anesthesia to HIV-infected women.
Pathophysiology
HIV, previously known as lymphadenopathy-associated virus (LAV) and human T-cell lymphotropic virus type III (HTLV-III), is a member of the lentivirus subfamily of human retroviruses. The lentiviruses typically cause indolent infections in their hosts. These infections are notable for central nervous system (CNS) involvement, long periods of clinical latency, and persistent viremia caused by an impaired humoral immune response.5 HIV is a retrovirus (i.e., it carries the enzyme reverse transcriptase). This enzyme converts the single-stranded viral RNA into double-stranded DNA, which subsequently can be integrated into the DNA of the infected cell. This process is error prone, leading to rapid mutation of the virus, which significantly complicates drug therapy. HIV displays similarity to human immunodeficiency virus type 2 (HIV-II), a virus that is endemic in western Africa and that produces a similar syndrome. HIV-II is even more closely related to the simian immunodeficiency virus (SIV). It has been suggested that HIV arose in human populations through transmission of SIV via infected “bush meat,” the meat of chimpanzees and other primates consumed as food.6
For infection of the host cell to occur, HIV must bind to a cell-surface receptor, the CD4+ antigen complex.7 This protein molecule was first detected on helper T cells, and it subsequently was identified on B cells, macrophages, and monocytes.8 It also is found on placental cells9 and may provide a route of vertical transmission to the fetus during early pregnancy. The interaction between HIV and host cells requires an interaction with an additional cell-surface protein; binding with either the CCR5 or CXCR4 co-receptor is required for infection to occur. A number of therapeutic agents target this interaction.10
Infection of helper T cells is the key to immune suppression in HIV disease. These cells play a major role in the initial recognition of foreign antigen as well as in the activation of other immune system components.11 CD4+ monocytes and macrophages are also targeted by HIV. In addition to these T cell–mediated effects, both neutropenia and disturbances of neutrophil function are common in the later stages of HIV infection.12 Abnormalities of these elements of the immune system render the HIV patient vulnerable to bacterial, viral, fungal, parasitic, and mycobacterial infection. In addition, for reasons that are not entirely clear, patients infected with HIV are susceptible to several malignancies (e.g., Kaposi’s sarcoma, B-cell lymphoma, invasive cervical carcinoma). AIDS-associated Kaposi’s sarcoma is almost exclusively limited to homosexual men with HIV or to women whose male sexual partners are bisexual; this fact suggests that the malignancy is related to another sexually transmitted disease. In fact, DNA sequences from human herpesvirus-8 have been identified in AIDS-associated Kaposi’s sarcoma.13,14
Diagnosis
The techniques for diagnosing HIV infection include viral culture, p24 antigen detection tests, nucleic acid amplification tests such as viral polymerase chain reaction (PCR), and immune function tests. Most often, the diagnosis is made on the basis of results of one of two antibody detection tests: enzyme immunoassay (EIA) and the Western blot technique. EIA measures the binding of anti-HIV antibody from the patient’s serum to a mixture of antigens that typically have been obtained through recombinant DNA techniques (third-generation tests). The use of third-generation tests has improved the reliability of EIA, but false-positive results (caused by autoimmune disorders, influenza or hepatitis B immunization, and/or high parity) and false-negative results (caused by immunosuppressive therapy and various malignancies) can occur.11 For these reasons, a Western blot test usually is performed after a positive EIA result is obtained. False-positive Western blot tests can also occur, but they are less common than false-positive EIA tests. The Western blot technique allows the identification of antibodies to nine specific HIV antigens. Different organizations have different criteria for a positive Western blot test, but a positive Western blot test generally requires the presence of antibody to at least three different antigens. If there is no detectable antibody to any of these antigens, the result is negative.11 Identification of any combination of antibodies that does not meet the criteria for a positive result is considered an indeterminate result and an indication for retesting in 4 to 8 weeks.
Nucleic acid amplification tests can detect extremely low levels of infection. This technique can detect viremia as early as 1 to 2 weeks after exposure, during the period of primary symptomatic infection.11 Although this technique can be used to diagnose acute HIV infection, it is typically used to monitor the response to ongoing antiretroviral therapy.
Both the EIA and the Western blot tests rely on the detection of antibody to HIV antigens. Unfortunately, there may be an interval of several weeks to months after the initial infection before detectable levels of antibody are present. A patient infected with HIV who is tested during this “window period” has a negative test result but is fully capable of infecting others. In fact, exceptionally high levels of viremia are present during the initial period of infection, and transmission after needle-stick injury or other occupational exposure is more likely.15 This is a strong argument for instituting universal precautions. If barrier precautions are instituted only for patients with positive test results, health care workers will be exposed unnecessarily to seronegative but infectious patients.
Patients may be chronically infected with HIV for many years yet appear clinically well or have only minor evidence of immune suppression, such as oral candidiasis or recurrent herpes zoster. The diagnosis of AIDS is made when any one of a number of AIDS-indicator conditions develops (Box 45-1).
Clinical Manifestations
In the early years of the AIDS epidemic, the predominant symptoms were those of immune suppression (e.g., opportunistic infections, unusual malignancies). Disturbances of gastrointestinal function were also prominent. As improvements in prophylaxis and treatment of opportunistic infections have increased longevity, it has become apparent that HIV eventually affects multiple organ systems. The aggressive use of highly active antiretroviral therapy (HAART) can significantly prolong the symptom-free interval, and it is highly unusual for a pregnant patient to have significant organ system involvement due to HIV.
Neurologic Abnormalities
Neurologic involvement can occur at any time during HIV infection (Box 45-2). Viral particles can be isolated from the cerebrospinal fluid (CSF) at the time of primary infection.16 The manifestations of nervous system involvement vary with the stage of the disease.
During initial systemic HIV infection, a variety of CNS disorders may occur. Headache, photophobia, and retro-orbital pain are common. Cranial and peripheral neuropathies, demyelinating polyneuropathy, and septic meningoencephalitis have been reported.17 Cognitive and affective changes (e.g., depression, irritability) may be noted. Most of these disorders are self-limited, but persistent neurologic dysfunction may occur.17
A subset of patients remains neurologically asymptomatic during the latent phase of HIV infection. Nevertheless, these patients typically have CSF abnormalities, including the local synthesis of HIV antibody and the presence of HIV particles or viral nucleic acid.18 This is an important consideration when one is determining the risk for introducing virus into the CNS during the performance of neuraxial anesthesia in an asymptomatic patient. It is almost certain that CNS infection has already occurred.
Finally, the late stages of HIV infection are marked by significant neurologic deterioration in almost all patients. Meningitis is common, and its causes include tuberculosis, infection with Cryptococcus, metastatic lymphoma, and direct infection of the meninges by HIV. Diffuse encephalopathy can occur; cytomegalovirus (CMV), herpes simplex virus (HSV), and toxoplasmosis typically produce a simultaneous impairment of both cognition and alertness. Diffuse encephalopathy may also be seen as a consequence of systemic disease, such as sepsis or hypoxemia secondary to respiratory disease. Patients with the AIDS dementia complex also have a diffuse encephalitic picture; however, unlike other forms of encephalitis in which cognitive function is diminished, the level of alertness remains unimpaired. In addition, the complex is associated with impairment of motor function and behavioral changes (apathy, agitation). Focal brain disorders can occur secondary to toxoplasmosis, primary CNS lymphoma, and progressive multifocal leukoencephalopathy, an opportunistic viral infection that causes selective destruction of white matter tracts. Myelopathy is common; it can manifest in an acute, segmental form, as in the transverse myelitis produced by varicella infection, or as a more progressive and diffuse disorder—vacuolar myelopathy—which is marked by a progressive, painless gait disturbance and spasticity. A distal, predominantly sensory peripheral neuropathy is quite common in late HIV infection. The etiology is unknown; it has been suggested to occur as a result of cytokine-mediated neurotoxicity.19 Sensory and motor dysfunction typically are minimal, but pain can be severe enough to prevent walking. CMV infection can also lead to a polyradiculopathy that usually responds to anti-CMV therapy. Autonomic neuropathy can manifest as mild postural hypotension or severe cardiovascular instability during invasive procedures. Autonomic dysfunction also can contribute to the chronic diarrhea that occurs in some patients with AIDS. An inflammatory myopathy resembling dermatomyositis has been reported, although this disorder is less common than the neuropathies.19 Finally, neurologic side effects of antiretroviral and other therapies also may occur (see later discussion).
Pulmonary Abnormalities
The pulmonary manifestations of HIV disease are caused not by a direct effect of the virus but, rather, by the opportunistic infections associated with the disease. The most prominent of these is Pneumocystis jiroveci (formerly known as Pneumocystis carinii), a fungal organism that is seen in a wide variety of mammals and appears to be carried asymptomatically by many humans.20 Despite this evidence of widespread exposure to the organism, symptomatic Pneumocystis pneumonia (PCP) is typically seen only in patients with severe immune suppression. The clinical picture is similar to the adult respiratory distress syndrome, consisting of severe hypoxemia and a pattern of diffuse interstitial infiltrates on chest radiography. The mortality rate of patients with PCP who require tracheal intubation may be as high as 75%.21 Early initiation of corticosteroid therapy decreases the likelihood of progression to respiratory failure.22 Patients who survive the disease are at risk for the development of pneumatoceles; subsequent rupture leading to pneumothorax is common. Survivors of PCP also are at risk for developing chronic airway disease, including chronic bronchitis and bronchiectasis.23
Reactivation of latent tuberculosis is common in patients with HIV infection because of the impairment of cellular immunity that ordinarily keeps the disease in a quiescent state; HIV-infected individuals also may be more susceptible to acquiring tuberculosis when they are exposed to an infectious individual.24 The impairment of humoral immunity is responsible for a higher incidence of bacterial pneumonia caused by encapsulated organisms (e.g., Streptococcus pneumoniae, Haemophilus influenzae).25 Finally, although less common than PCP, pneumonia secondary to other fungal organisms (e.g., Aspergillus, Cryptococcus, Coccidioides) is much more common in patients infected with HIV than in the general population.25
Gastrointestinal Abnormalities
Gastrointestinal disturbances occur at some time in almost all patients with HIV infection (Box 45-3). Painful or difficult swallowing is common and is typically caused by herpetic, CMV, or candidal esophagitis; the contribution of these disorders to gastroesophageal reflux is unclear.26 Severe diarrhea resulting from infection with CMV, HSV, Shigella, Salmonella, Candida, Cryptosporidia, Giardia, Mycobacterium avium complex (MAC), or HIV itself can lead to significant cachexia and electrolyte abnormalities. Finally, hepatobiliary disease is common. Causes of parenchymal liver disease include hepatitis B and C, CMV, mycobacterial infection (both Mycobacterium tuberculosis and MAC), and Cryptococcus. Kaposi’s sarcoma and non-Hodgkin’s lymphoma may involve the liver. Biliary tract disease can develop in patients with advanced HIV infection; although several pathogens have been associated with this disorder (cryptosporidium, CMV), treatment of those pathogens is seldom effective.27 Endoscopic retrograde cholangiopancreatography (ERCP)–guided stenting has been successfully used to treat this disorder.28
Hematologic Abnormalities
HIV infection is associated with hematologic abnormalities that affect each of the peripheral cell lines.12 Leukopenia is a hallmark of the disease, especially the depletion of CD4+ lymphocytes; qualitative alterations in the functions of neutrophils and macrophages also occur. Anemia is quite common. Causes include direct HIV infection of erythroid precursors, suppression of erythropoiesis due to inappropriate release of tumor necrosis factor, infiltration of bone marrow with MAC or malignancy, and occult gastrointestinal blood loss.
Coagulation disturbances are common in patients with HIV. Immune thrombocytopenia is common and typically is only mildly symptomatic. Platelet production may be impaired because of direct infection of megakaryocytes with HIV. Thrombocytopenia frequently responds to the initiation of antiretroviral therapy. The response to corticosteroid therapy is variable. Intravenous immune globulin produces a rapid but transient effect, and it may be indicated in patients with life-threatening hemorrhage. The activated partial thromboplastin time may be prolonged because of the presence of the lupus anticoagulant; this finding is linked to a higher incidence of major thromboembolic events in HIV-infected patients. Finally, many of the antiretroviral agents and other drugs used in these patients have hematologic toxicity.
Cardiovascular Abnormalities
When echocardiography and autopsy evidence of lymphocytic infiltration of the myocardium are used as evidence of cardiovascular involvement in patients with HIV, the prevalence of such involvement is as high as 50%.29 Nevertheless, clinically significant cardiovascular disease is rare in patients with HIV. Pericarditis has been reported to be the most prevalent cardiovascular disorder seen in HIV-infected patients. The most common etiology appears to be mycobacterial infection; CMV, HSV, Kaposi’s sarcoma, malignant lymphoma, and HIV itself have also been implicated.30 Pulmonary hypertension can develop secondary to repeated episodes of PCP and can also be a consequence of cytokine-mediated endothelial injury.29 Direct myocardial involvement—typically, focal myocarditis—is identified in 15% to 50% of autopsy studies, but clinical myocarditis or cardiomyopathy is rare.29 Infective endocarditis among patients with HIV occurs almost exclusively in intravenous drug users. Finally, the elevations in serum cholesterol and triglyceride concentrations produced by antiretroviral agents appear to increase the risk for coronary artery disease in patients receiving these drugs.31
Endocrine Abnormalities
Endocrine dysfunction can result from HIV infection, opportunistic infections, or drug therapy.32 There is a relatively high incidence of pathologic findings in the adrenal gland at autopsy, yet clinical evidence of glucocorticoid insufficiency is rare. Patients with AIDS frequently have abnormal thyroid function test results, similar to the findings in patients with other chronic illnesses, yet clinical hypothyroidism is unusual. Insulin resistance and diabetes are increasingly recognized as consequences of HIV infection and antiretroviral treatment.33
Renal Abnormalities
Patients with HIV are at risk for acute renal failure secondary to sepsis, dehydration, and drug toxicity.34 A common cause of chronic renal insufficiency is proliferative glomerulonephritis secondary to deposition of immune complexes containing HIV antigen within the glomeruli. Renal failure may also occur because of a specific disorder, HIV-associated nephropathy.35 This entity, seen almost exclusively in African-American patients, is characterized by a focal segmental glomerulosclerosis. Hypertension is uncommon, deterioration of renal function is extremely rapid, and the long-term prognosis is worse than that seen in renal failure from other causes. The underlying cause appears to be direct infection of renal cells by HIV. Antiretroviral therapy appears to modify the course of the disease.35
Interaction with Pregnancy
In 1991 and in 1995, the nationwide seroprevalence rate of HIV during pregnancy was reported to be 1.5 and 1.7 per 1000 pregnant women, respectively.36,37 There was considerable geographic variation in these figures; the highest rates of seroprevalence were found in New York (5.8 per 1000), Washington, DC (5.5 per 1000), and New Jersey (4.9 per 1000). In 1991, seropositive women were identified in all but 2 of the 39 reporting areas.36 In New York City, the prevalence of HIV infection among pregnant women was 6.2 per 1000 in 1999-2000, having declined by 49% over the previous decade. This decrease was markedly greater in white women than in African-American women.38
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


