HIV Infection
INTRODUCTION
Perinatal transmission is the most common route of human immunodeficiency virus type 1 (HIV-1) infection among infants and children.1 Since the first reports of pediatric acquired immune deficiency syndrome (AIDS) cases more than 3 decades ago,2 extraordinary advances have occurred in the prevention and treatment of pediatric HIV infection.3–9 The epidemiology of the perinatal HIV epidemic has dramatically changed in the United States and other resource-rich countries because of effective implementation of strategies to prevent vertical transmission.7–9 Improved survival of HIV-infected children into adolescence and adulthood because of the availability of highly active antiretroviral therapy (HAART) has significantly improved the health and longevity of HIV-infected children.3–6
Preventing perinatal HIV transmission became a reality in 1994 when the Pediatric AIDS Clinical Trials Group (PACTG) 076 published data showing that a long complex course of zidovudine (ZDV) prophylaxis given to an HIV-1-infected mother during early gestation and labor and then postnatally to the baby reduced perinatal HIV-1 transmission by almost two-thirds.10 In 1995, the US Public Health Service (USPHS) issued guidelines recommending universal counseling and testing for pregnant women and use of ZDV to reduce perinatal transmission.11 Since then, rates of perinatal HIV transmission in the United States and Europe have decreased to less than 1%–2% because of widespread implementation of universal antenatal HIV testing, combination antiretroviral treatment (ART) during pregnancy, elective cesarean section, and avoidance of breast-feeding through the use of formula milk.1,12–14 Currently, new pediatric HIV infections are rare in the United States and occur primarily because of missed prevention opportunities.15 In recent years, remarkable progress has occurred in the prevention of mother-to-child HIV transmission (PMTCT) during breast-feeding.16–18 However, translation of research into policy and practice remains a major challenge in many low- and middle-income countries (LMIC).16–20
This chapter reviews the epidemiology of perinatal HIV infection; discusses the pathophysiology of transmission; reviews the diagnosis, differential diagnosis, and clinical manifestations; describes the treatment and outcomes; and briefly summarizes the current perinatal HIV preventive strategies focusing primarily in the United States. Review of the global advances in PMTCT is outside the scope of this chapter, and they are not discussed; excellent reviews have been published on this topic.1,8,16–18
EPIDEMIOLOGY
Global Scope of the Problem
The World Health Organization (WHO) progress report in 2011 estimated that 34 million (31.6–35.2 million) adults worldwide were living with HIV/AIDS.20 Approximately 2.7 million (2.4–2.9 million) adults had become newly infected with HIV in 2010 and 1.8 million (1.6–1.9 million) HIV-infected individuals died. In sub-Saharan Africa, approximately 22.9 million inhabitants were living with HIV.20 In South and Southeast Asia, roughly 4 million people are living with HIV, followed by the Americas, including the Caribbean, where approximately 3 million HIV-infected individuals reside.20
The global pediatric HIV epidemic is driven by high levels of endemic HIV infection in women living in sub-Saharan Africa. Worldwide, 49% (46%–51%) of HIV-positive adults are women of childbearing age. In sub-Saharan Africa, 6 in 10 adults living with HIV in 2011 were women.21 Seroprevalence rates among pregnant women have exceeded 35% in some antenatal clinics in several countries in sub-Saharan Africa, including South Africa, Swaziland, Lesotho, and Botswana.20 Roughly 80% of HIV-infected women reside in sub-Saharan Africa.20 HIV/AIDS is a major cause of morbidity and mortality among women of childbearing age in sub-Saharan Africa.
In 2011, approximately 3.4 million (3.1–3.9 million) children younger than 15 years were living with HIV; around 330,000 (280,000–380,000) infants became newly infected with HIV in 2011 alone, and an estimated 230,000 (200,000–270,000) children died from AIDS-related illness in the same year.21 On a daily basis, roughly 1068 babies become infected with HIV, primarily through mother-to-child HIV transmission (MTCT) during pregnancy, labor, and delivery or postnatally during breast-feeding.16,17 Roughly 90% of HIV-infected children live in sub-Saharan Africa.20
HIV/AIDS in Women
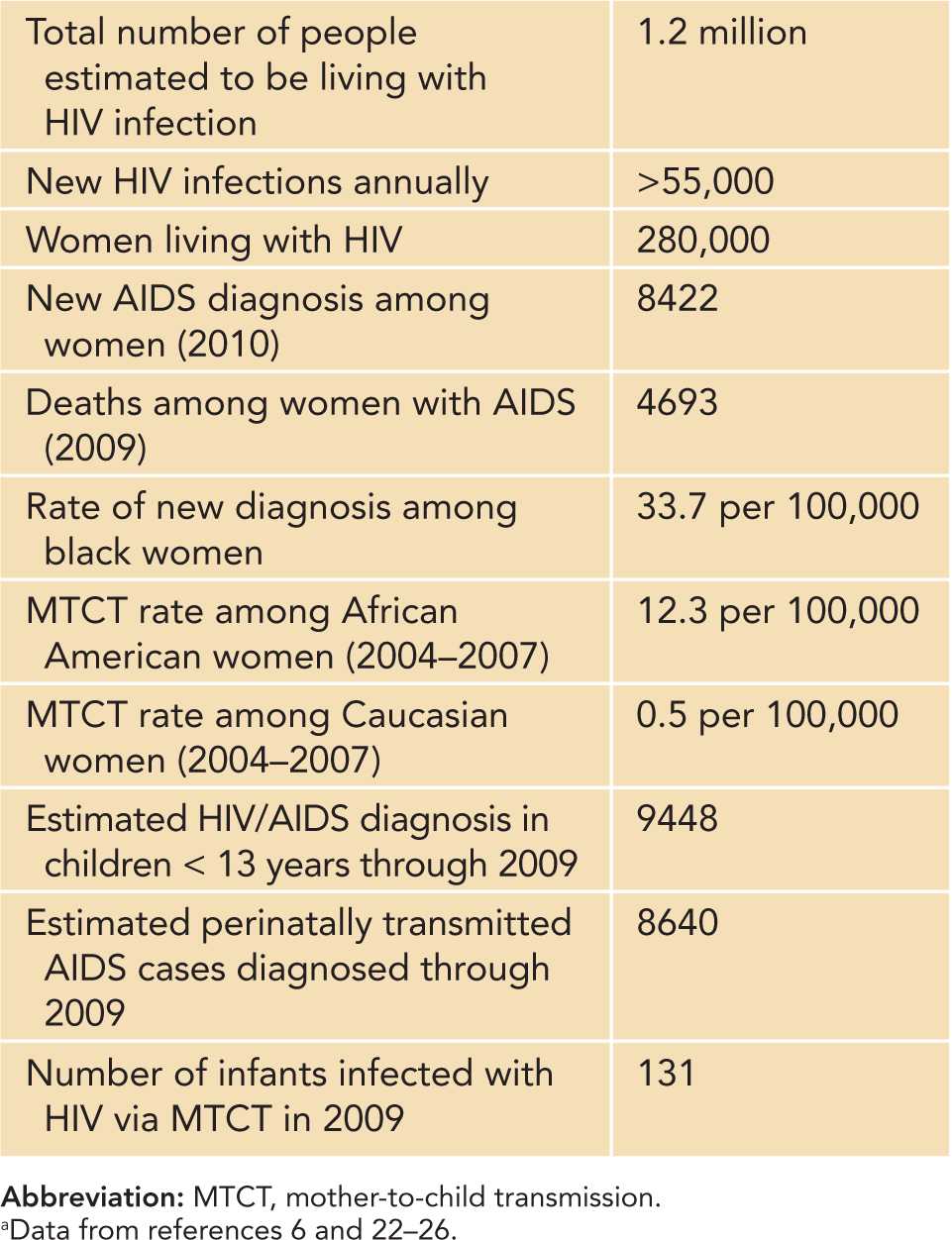
Since the beginning of the HIV epidemic in the United States, men accounted for the majority of new HIV infection and AIDS diagnoses. However, the incidence of HIV infection among women increased gradually until the late 1980s, but then declined in the early 1990s and has remained relatively stable over the past decade.22,23 Of reported AIDS cases in adults, women accounted for 8% in 1985, 13% in 1993, 20% in 1995, 23% in 1999, 27% in 2000, and 25% in 2010.22 Currently, approximately 1.2 million individuals in the United States are living with HIV/AIDS, including nearly 280,000 women (Table 58-1).24,25 One in 5 (20%) of HIV-positive individuals are unaware of their infection. In 2009, there were 11,200 new HIV infections, and in 2010, there were 8422 new AIDS diagnoses among women.22,23,26 In 2009, among women with AIDS, 4,693 deaths occurred.22 HIV infection was among the top 10 leading causes of death for black/African American females aged 10–54 and Hispanic/Latino females aged 15–54.
Table 58-1 US Epidemiology of Adult and Pediatric HIV Infectiona
Women of color, especially black/African American and Hispanic/Latino women are disproportionately affected by the HIV/AIDS epidemic. In 2010, of the total number of new HIV diagnoses, 64% occurred in black/African American females (representing 12% of the US female population), followed by 16% in Hispanic/Latino females (representing ~ 14% of the female population). In contrast, white females (representing 68% of the female adult and adolescent population) accounted for only 18% of new HIV diagnoses among females. More than 75% of women with AIDS are in the reproductive age group at the time of diagnosis.
At the end of 2009, areas with the highest estimated rates (per 100,000 population) of adult and adolescent females living with a diagnosis of HIV infection were the US Virgin Islands (497.2), New York State (464.9), Florida (353.3), Puerto Rico (337.6), and New Jersey (330.8). Regional HIV seroprevalence rates vary, with the highest rates found among women residing in the northeastern and southern states. In 2009, the top 10 states with the highest number of women/girls living with AIDS were New York (n = 23,859) followed by Florida (n = 15,081), California (n = 7817), Texas (n = 6795), New Jersey (n = 6312), Maryland (n = 6080), Pennsylvania (n = 4961), Georgia (n = 4679), Illinois (n = 3638), and Puerto Rico (n = 3326).25,26 During 2010, the highest rates of new AIDS diagnoses (per 100,000 population) among adult and adolescent females were in the District of Columbia (79.9), Maryland (18.5), and Louisiana (15.5), followed by Florida (14.7), the US Virgin Islands (14.3), New York (14.1), New Jersey (11.6), Delaware (10.9), and Mississippi (10.3).25,26
For women living with HIV, the most common mode of transmission is high-risk heterosexual exposure and injection drug use. Among black and Latino women, heterosexual transmission accounts for 85% and 82% of new HIV/AIDS diagnoses, respectively, compared to white women (72%).24 Injection drug use accounts for a greater proportion (28%) of new infections among white women.24 Although dramatic decline (<2%) in the rate of perinatal HIV transmission has been documented in the United States and the relatively stable incidence of HIV among women, perinatal HIV infection will continue to occur each year, primarily among young black women warranting close surveillance.1,22 A number of social factors, such as poverty, tight social networks, and assortative mixing as well as lack of prenatal care, antenatal HIV testing, and early access to combination ART contribute to the high rates of HIV infection among black women in the United States.27
HIV/AIDS in Children and Adolescents
Since the mid-1990s, the annual number of diagnoses of perinatally acquired HIV/AIDS cases has declined by more than 96% in the United States as a result of routine antenatal HIV testing in conjunction with implementation of effective interventions to prevent transmission.28,29 In 1991, the annual number of infants with perinatal-acquired HIV peaked at 1650 infants but significantly declined to an estimated 215–370 cases in 2005, to 182 cases in 2008, and to 131 cases in 2009 (Table 58-1).6,29–31 Cases of AIDS in children have accounted for less than 1% of all reported AIDS cases in the United States.6
The racial and ethnic and geographic distribution of AIDS cases in children parallels that of women with AIDS. Racial/ethnic disparities in perinatal HIV/AIDS incidence have persisted since the early part of the epidemic; 78% of children with AIDS were black or Hispanic30 in 1981–1986. From 2004 through 2007, the Centers for Disease Control and Prevention (CDC) estimated that the overall annual rate of diagnosis of perinatal HIV infection in the 34 states was 2.7 per 100,000 infants aged less than 1 year.31 Perinatally acquired HIV infection has occurred more frequently (85%) among black or Hispanic children during the same time period.31 Compared to white children, rates were 23 times higher among black and 4 times higher among Hispanic children. However, there was a noted decrease in the racial/ethnic disparity during 2004–2007, as the annual rate of diagnosis of perinatal HIV infection decreased from 14.8 to 10.2 per 100,000 population for black infants.31 Likewise, the rate of diagnosis of perinatal infection decreased from 2.9 to 1.7 per 100,000 population for Hispanic infants during the same time period.31
The rate of acquisition of HIV/AIDS among adolescents and young adults continues to increase in the United States, occurring primarily among populations of minority race or ethnicity. Young black men who have sex with men (MSM) are at highest risk; during 2006–2009, new HIV cases increased by 48% in young US MSM.23 Infection among adolescent women is acquired primarily through heterosexual contact.23 In 2007, of adolescents and youth aged 13–19 years of age diagnosed with HIV, 31% were females, compared with 23% of individuals aged 20–24 years and 26% of adults aged 25 years or older.6 In 2007, cases of AIDS in adolescents and young adults aged 13–24 years accounted for 4% of people living with HIV infection in the United States.6
Other modes of transmission of HIV, such as exposure to contaminated blood products, have been virtually eliminated because of effective screening methods since 1985 in the United States.6 Probable HIV transmission from HIV-infected caregivers to their infants via feeding blood-tinged premasticated food has been reported, but no transmission of HIV infection to household contacts through casual contact has been documented.6
PATHOPHYSIOLOGY
Virus Biology
Regarding virus biology, HIV-1 is an enveloped cytopathic virus belonging to the Lentiviridae family of retroviruses and has a complex genomic structure, closely related to the simian immunodeficiency viruses (agents in African green monkeys and sooty mangabeys).32,33 HIV-1 variants are classified into 3 distinct genetic groups: group M (main), group O (outlier), and group N (non-M/non-O). Group M accounts for the majority of HIV-1 infections globally and is further divided into 10 subtypes or clades (A to K). Individuals who acquire HIV-1 infection in the United States, Western Europe, and Australia are most frequently infected with subtype B.32 Other HIV-1 subtypes circulate globally. Subtypes C and D predominate in southern and eastern Africa, subtype C on the Indian subcontinent, and subtype E is common in Southeast Asia.
Like all retroviruses, HIV-1 contains 3 principal genes: (1) gag, which encodes the core nucleocapsid polypeptides (gp24, p17, p9); (2) env, which encodes the surface-coat proteins of the virus (gp120 and gp41); and (3) pol, which codes for the viral reverse transcriptase and other enzymatic activities (ie, integrase and protease). There are 2 regulatory (tat and rev) and 4 accessory genes (vif, vpr, vpu, and nef) that are essential for viral assembly and release. The retroviral core also contains 2 copies of the viral single-stranded RNA that requires the activity of a viral enzyme, reverse transcriptase, to convert the viral RNA to DNA.34 A double-stranded DNA copy of the viral genome then incorporates into the host cell genome, where it persists as provirus.
The life cycle of HIV-1 is characterized by several distinct stages.33,34 The first step in the entry process of HIV into a cell is the binding of the virion envelope glycoproteins (gp120 and gp41) to CD4 on resting or activated T cells. This results in conformation change in the envelope, interaction with a coreceptor, and fusion of the viral and cell membranes, allowing the viral genome to gain entry into the cell. Members of the chemokine receptor family are coreceptors for HIV. Human cord blood mononuclear cells are preferentially infected by macrophage-tropic (M-tropic) strains of HIV-1 using the CC chemokine receptor CCR5. T cell–tropic strains replicate in CD4+ T cells and macrophages and use the chemokine receptor CXCR4, a member of the CXC chemokine family.33,34
Rates, Timing, and Mechanisms of Transmission
Mother-to-child transmission of HIV can occur during pregnancy, during labor and delivery, or postnatally through breast-feeding.1,16,17,35 In the absence of antiretroviral (ARV) and obstetric interventions, the perinatal HIV transmission rate is estimated to be 15% to 25% in a resource-rich setting (eg, United States, Europe).35 In contrast, without intervention, MTCT rates of 25%–45% has been observed among breast-feeding populations in Africa.35 Knowledge about the precise timing of transmission is crucial for developing innovative preventive interventions.36 Data based on cord blood or newborn HIV polymerase chain reaction (PCR) testing indicate that most (50% to 60%) of the perinatal HIV transmission occurs around the time of labor and delivery.37 An infant is considered to have been infected in utero if the HIV-1 genome can be detected by PCR or be cultured from blood within 48 hours of birth. In contrast, a child is considered to have intrapartum infection if diagnostic assays such as culture, PCR, and serum p24 antigen were negative in blood samples obtained during the first week of life but became positive during the period from day 7 to day 90 and the infant had not been breast-fed.38 In the breast-fed infant, 20%–25% of HIV transmission occurs during pregnancy, 35%–50% during labor and delivery, and another 25% to 35% of transmission during lactation.35
Intrauterine Infection
Early reports using PCR and in situ hybridization technology indicated the possibility of in utero transmission because the HIV was detected in aborted fetal tissues and amniotic fluid obtained during the first and second trimesters of pregnancy.39 However, subsequent studies in animals and human fetuses reported almost no transmission during the first and second trimesters of pregnancy.40,41 Based on viral detection during the first 48 hours of birth, intrauterine transmission occurs in about 20%–25% of infections.35 Statistical modeling data also suggest that most in utero HIV transmission occurs during the last few weeks before delivery when the vascular integrity of the placenta is disrupted.1,42
Intrapartum Infection
Intrapartum transmission may occur in a variety of ways, including direct exposure of the infant with infected maternal blood and cervicovaginal secretions during birth, ascending infection after rupture of membranes, or maternal-fetal microtransfusions during uterine contractions.43,44 Intrapartum transmission is supported by studies failing to detect HIV in infants born to HIV-infected women in the first month of life but subsequent detection of virus after 1 to 3 months of life.45 Additional evidence to support exposure to maternal virus during delivery as a likely route of transmission include findings of increased HIV infection rates in first-born twins, increased risk associated with prolonged rupture of membranes, and the protective effect of elective cesarean delivery before onset of labor.46–49 Some reports have documented an increased risk of MTCT associated with placental malaria infection, but not others.50,51
Postnatal Infection
In LMIC, where breast-feeding is the cultural norm and safe replacement feeding is not affordable, feasible, sustainable, or safe, postnatal transmission of HIV during breast-feeding remains a significant concern.16–18,52,53 The exact mechanisms of breast milk HIV transmission are poorly understood. HIV genomes have been isolated from cellular and cell-free fractions of human milk from HIV-infected women and have been detected by culture or PCR in varying frequencies (39%–89%) in many studies.54,55 Cell-associated virus may be a stronger predictor for transmission of HIV to the infant than cell-free virus.54 Transmission of HIV through breast-feeding accounts for up to one-third to one-half of all new pediatric HIV infections. An estimated transmission risk of 10%–15% of all HIV-exposed infants exists; infants are infected through prolonged breast-feeding.35,53 A large individual patient data meta-analysis from sub-Saharan Africa suggested that the risk of postnatal HIV transmission (after 4 weeks of life) is substantial and relatively constant (~0.7% per month of breast-feeding).56
Premastication
Anecdotal case reports from the United States have described probable HIV transmission by caregivers who premasticated food for infants.6 Transmission in the reported cases was likely caused by blood-borne virus in the saliva (and not salivary virus) because bleeding gums or sores were described in 2 of the caregivers. Because safe alternative feeding methods are available in the United States, the CDC recommends physicians to counsel HIV-infected caregivers not to premasticate food for infants.6
Risk Factors
Many risk factors for perinatal HIV transmission have been identified, including maternal, obstetric, infant, genetic, and viral-related characteristics (Table 58-2).36,57,58 The most critical factor associated with increased risk of intrauterine and intrapartum transmission is the maternal plasma HIV viral load.13,57,58 However, perinatal transmission can occur across the entire range of maternal viral load among pregnant women (including those with low or undetectable serum levels of HIV around the time of labor and delivery).59 Other maternal risk factors for perinatal HIV transmission include advanced clinical disease, acute HIV infection during pregnancy, and low CD4 T-lymphocyte counts. HIV viral load in cervicovaginal secretions is an independent risk factor for perinatal HIV transmission.60 Genital ulcer diseases, especially herpes simplex virus (HSV), and syphilis, and other coinfections (such as hepatitis C virus, hepatitis B virus, malaria, tuberculosis) may increase the risk.8,61–63 Behavioral risk factors, including maternal substance abuse, cigarette smoking during pregnancy, and noncompliance to ART, may also increase the risk of transmission.64 Obstetric risk factors associated with increased risk of transmission include vaginal delivery, prolonged rupture of membranes, chorioamnionitis, and invasive obstetric procedures.65 Premature infants born to HIV-infected women have a higher rate of perinatal HIV infection than full-term infants.48
Table 58-2 Risk Factors for Perinatal HIV Transmission
Maternal
• High maternal HIV viral load in plasma (and breast milk in breast-feeding populations)
• Low maternal CD4 T-lymphocyte count
• High vaginal/cervical shedding of HIV
• Advanced maternal clinical disease
• Concurrent STIs (syphilis, genital HSV)
• Coinfections (TB, malaria, HBV, HCV)
• Behavioral (cigarette smoking, substance abuse, poor adherence to combination ART)
• Genetic and immunologic characteristics (maternal HLA-A2301, upregulation of CCR5 receptor expression in placenta)
Obstetric
• Invasive procedures (amniocentesis, invasive monitoring)
• Chorioamnionitis
• Prolonged rupture of membranes
• Vaginal delivery (VL > 1000 copies/mL)
Fetal/Infant
• Prematurity (<34 weeks)
• Twin gestation (higher infection rate in first-born twin)
• Low birth weight
• Infant feeding choice (breast-feeding duration, mixed infant feeding)
• Maternal breast disease (mastitis, cracked or bleeding nipples)
• Genetic and immunologic characteristics (innate immunity/β-defensin polymorphisms, mannose-binding lectin gene polymorphisms, genetic variants of chemokine and chemokine receptors)
Abbreviations: ART, antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HSV, herpes simplex virus; STI, sexually transmitted infection; TB, tuberculosis; VL, viral load.
Besides maternal viral load, other viral intrinsic factors affecting transmission include viral subtype, recombinant forms, resistant viral strains, and replication fitness.16,52 Genetic and phylogentic studies indicated that infant quasi species are highly homogeneous and generally represent minor maternal variants, confirming that vertical transmission of clade B HIV transmission occurs across a selective bottleneck.66 In this study, infant clones did not differ from the maternal clones in env length or glycosylation, and all infant variants utilized the CCR5 coreceptor but were not M-tropic.66 Preferential in utero transmission of HIV subtype C compared to subtype A or D has been reported.67 Host factors, including maternal-infant HLA concordance and maternal HLA homozygosity have been associated with increased transmission risks in MTCT, whereas genetic variants of chemokine and chemokine receptors have yielded conflicting results.68,69
Risk factors for breast milk HIV transmission include viral factors (high HIV DNA or RNA level in plasma and breast milk, maternal primary infection during lactation, decreased maternal CD4 cell count, maternal symptomatic disease/AIDS, virus subtype, and recombination forms); maternal clinical or immunological factors (bleeding or cracked nipples, subclinical/clinical mastitis, breast abscesses, malnutrition, micronutrient deficiencies); type of infant feeding (prolonged breast-feeding, mixed infant feeding); and maternal and infant host factors (oral candidiasis, oral ulcers).57,58
Pathogenesis of Perinatal HIV Infection
Recent studies have improved our understanding of the molecular mechanisms of MTCT. Although progress has been made in the understanding of the viral and immunopathogenesis of perinatal HIV infection, the true correlates of immune protection and immune failure and mechanisms of infection in neonatal target cells in the context of extreme viral and HLA diversity are unknown.70–72 Compared to adult cells, HIV replicates more readily in neonatal T lymphocytes and monocytes or macrophages.72 The env glycoprotein (gp160) engages the HIV receptor and coreceptors, facilitating the entry of the virus into cells, and represents the primary target for neutralizing antibodies. Viruses isolated in early vertical infection predominantly use the CCR5 coreceptor, although use of other receptors has been reported. The activation and direct infection of CD4 T cells result in high rates of viral production and dissemination throughout the body.
Perinatal HIV infection is characterized by high levels of plasma RNA (often exceeding 105–107/mL), decreasing only slowly to a “set point” by approximately 2 years of age, and may be related to many factors, including the increased HIV gene expression and replication in neonatal target cells, a large and renewable CD4 cell pool size, presence of an active thymus, and delayed or ineffective HIV-specific immune responses.70–72 High viral loads correlate with more rapid disease progression in neonates or infants than adults, while lower levels of plasma HIV RNA with HAART are associated with clinical benefit. Regardless of the route of infection, the gastrointestinal lymphoid tissues are a major site of viral replication, persistence, and loss of CD4 T lymphocytes throughout infection.73
Immune disturbances in HIV infection lead to severe deficiencies in cell-mediated and humoral immunity resulting from quantitative and qualitative defects. In infants, thymic injury from HIV infection may have a significant impact on the developing immune system, resulting in progressive depletion of thymic CD4 T-lymphocyte cells, dramatic decrease in cortical CD4/CD8 double-positive cells, and an increased percentage of CD8 cells.72 Other immune abnormalities noted include an inverted ratio of CD4/CD8, polyclonal B cell activation resulting in hypergammaglobulinemia (especially IgG and IgA), decreased lymphocyte proliferation in response to an antigen, and altered function of monocytes and neutrophils.72 Panhypogammaglobulinemia is noted in fewer than 10% of patients and is associated with poor prognosis.6
HIV-Specific Immune Response
Vertically infected infants face considerable challenges in generating a specific immune response to HIV.70 First, HIV transmission occurs before the immune system is fully developed in the infant, allowing for more efficient viral replication and less-efficient immunologic containment of the virus.70 Second, infected infants carry a high frequency of maternally inherited HLA class I alleles (such as HLA-B*1801 and B*5802) associated with poor control of HIV. Third, transmitted maternal escape mutants are adapted to maternal HLA alleles and therefore preadapted to an infant’s HLA.74 Fourth, passive transfer of maternal, nonneutralizing antibodies could inhibit development of HIV-specific immune responses.75
CD8 cytotoxic T lymphocytes (CTLs) play a critical role in generation of HIV-specific immune response in acute adult infection. Many CTL epitopes are identified in various HIV genes that are conserved in HIV mother-infant sequences, indicating a role in perinatal transmission.72 Although HIV-specific CTL activity can be demonstrated at a very early age, even in the fetus, the response is weak, less broad, and not associated with reduction in viral load and clinical outcomes compared to adults with primary infection.76 HIV-specific CTL responses become more frequent and broad in infected infants after 6 months of life.76 Some CTL responses in infants can select for viral escape variants very early in life.77 In addition, disease progression is slower in children who express HLA-B*27 or HLA-B*57, indicating that CTL responses can have an important role in suppression of HIV in pediatric infection.70 After the decline of passively transferred maternal antibody-dependent cellular cytotoxicity (ADCC) antibodies, the production of HIV-envelope cytotoxic antibodies is delayed in vertically infected infants.78 Although neutralizing antibodies can be generated during early infection, the precise role of neutralizing antibodies in limiting MTCT is unclear.70
Several studies indicated that HIV-infected children have reduced antibody responses to certain childhood vaccines (eg, diphtheria, acellular pertussis vaccine).78 Reduced antibody responses following immunization and vaccine failures in HIV-infected infants may result from a poor primary immune response, failure to generate memory responses, or loss of memory cells.78 The majority of vertically infected infants who receive HAART before 3 months of age develop antibody and lymphproliferative responses to routine infant vaccines, although persistent HIV-specific immune responses are not detected.78
DIFFERENTIAL DIAGNOSIS
Human immunodeficiency virus (HIV) infection in children and adolescents causes a wide range of clinical manifestations, from asymptomatic infection to marked immunodeficiency. HIV infection must be included in the differential diagnosis of a wide spectrum of pediatric disorders (Table 58-3).
Table 58-3 Differential Diagnoses of HIV Infection
• Anemia, chronic
• Autoimmune and chronic benign neutropenia
• Primary immune deficiency syndromes (Bruton agammaglobulinemia, common variable immunodeficiency, severe combined immunodeficiency, transient hypogammaglobulinemia of infancy)
• Constitutional growth delay
• Failure to thrive/malnutrition
• Lymphadenopathy
• Malabsorption syndromes
Clinical Manifestations
The clinical features of HIV infection in infants are highly variable and often nonspecific. Infants with perinatally acquired HIV infection are often asymptomatic, and physical examination is usually normal in the neonatal period. Growth delay can be an early sign of untreated perinatal HIV infection. Other features of infection in early infancy could include unexplained persistent or recurrent fevers or generalized lymphadenopathy, often associated with hepatosplenomegaly and recurrent or persistent otitis media (Table 58-4). Also commonly encountered are oral or diaper candidiasis, developmental delay, parotitis, and dermatitis.6
Table 58-4 Clinical Manifestations of HIV Infection in Infants Ages 1 to 2 Years
Failure to thrive, HIV wasting syndromea
HIV encephalopathya
Hepatosplenomegaly, lymphadenopathy
Pneumocystis jiroveci pneumoniaa
Multiple or recurrent bacterial infections (especially Streptococcus pneumoniae)a
Refractory thrush or candida diaper dermatitis
Other opportunistic infections (eg, cytomegalovirus disease, severe herpes simplex virus)a
Lymphoid interstitial pneumonia or pulmonary lymphoid hyperplasia complexa
aAIDS-defining condition.
HIV encephalopathy is a well-described AIDS-defining complication of HIV in infants and children and can affect 10% to 25% of cases.79,80 Encephalopathy can be either static or progressive, characterized by a classic triad of developmental delay, impaired brain growth or acquired microcephaly, and acquired symmetric motor deficits (hyperreflexia, hypertonia, ataxia, or gait disturbances).81 Characteristic computed tomographic findings characteristic of HIV encephalopathy include cerebral atrophy (85% of cases) and bilateral symmetric calcification of the basal ganglia (15% of cases). Criteria for the definitive diagnosis of HIV encephalopathy are listed in Table 58-5. The onset of HIV encephalopathy is in the first year of life and was associated with increased mortality in the pre-ART era.82 In the current era of HAART, the incidence of HIV encephalopathy decreased by 50%, but infected infants may present with more subtle and insidious central nervous system (CNS) manifestations.79,83,84
Table 58-5 Clinical and Neuroimaging Features of HIV Encephalopathy in Infantsa
Clinical Manifestations
• Acquired microcephaly
• Developmental delay or regression
• Spasticity
• Pathologic reflexes
• Dystonia
• Gait abnormalities
• Expressive language impairment
Neuroimaging Features
• Cerebral atrophy
• Symmetric calcifications in basal ganglia
aData from reference 81.
In the pre-ART era, Pneumocystis jiroveci pneumonia (PCP) (previously known as Pneumocystis carinii) was the leading AIDS-defining illness diagnosed during the first year of life and was associated with a high mortality rate. Other common AIDS-defining conditions in US children with vertically acquired infection include lymphoid interstitial pneumonitis, multiple or recurrent invasive bacterial infections caused by encapsulated bacteria, HIV encephalopathy, wasting syndrome, candida esophagitis, cytomegalovirus (CMV) disease, and Mycobacterium avium complex (MAC) infection.81 With early infant HIV diagnosis and linkage to care and an ART program, the frequency of historically reported AIDS-defining illness and opportunistic infections (OIs) have dramatically decreased among children living with HIV in the United States and other resource-rich countries.85 In the post-ART era, the rate of OIs decreased from 12.5 to 0.8 cases per 100,000 person-years pre- and post-ART, respectively.85
Pediatric AIDS Case Definition
The AIDS case definitions published by the CDC in 1987, and subsequently revised in 1993, 1994, and 2008, are intended primarily for public health surveillance and reporting purposes for monitoring the HIV epidemic.86–89 The revised 1994 CDC classification system for HIV infection in children less than 13 years of age is based on (1) HIV infection status, (2) clinical disease, and (3) immunologic status.88 Clinical categories are stratified from N, indicating no signs or symptoms, through A, B, and C, for mild, moderate, and severe (AIDS-defining) symptoms, respectively. The revised 2008 Pediatric AIDS case definitions did not make any changes in the HIV infection classification system, the 24 AIDS-defining conditions for children aged less than 13 years, or the AIDS case definition for children aged less than 18 months.89 The case definitions are similar for adults and children with some important exceptions.88 Lymphoid interstitial pneumonia (LIP) and multiple or recurrent serious bacterial infections are AIDS-defining illnesses only for children. Also, certain herpes virus infections (CMV, HSV) and toxoplasmosis of the CNS are AIDS-defining conditions only for adults and children older than 1 month of age.88
The immunologic categories place emphasis on the CD4 T-cell lymphocyte count and percentages for age, and include stage 1, no evidence of immunosuppression; stage 2, moderate immunosuppression; and stage 3, severe immunosuppression.88,89 Once classified, a child cannot be reclassified into a less-severe category, even if the child’s clinical status or immune function improves in response to ART or resolution of clinical events. HIV-exposed infants whose HIV infection status is indeterminate (unconfirmed) are classified by placing a prefix E (for perinatally exposed) before the appropriate classification code (eg, EN2).89
DIAGNOSTIC TESTS
Early Infant Diagnosis
Many advances have been made in the area of laboratory diagnosis of HIV infection.90–92 Routine HIV antibody testing is not informative for early infant diagnosis because of transplacental passage of maternal IgG antibodies to the virus that are present in infants up to 18 months of age.6 The diagnosis of HIV infection in infants warrants the use of PCR-based DNA or RNA assays (referred to as HIV nucleic acid amplification tests, NAATs) that are highly sensitive and specific and now widely available in developed countries.90
The HIV DNA PCR assay detects cell-associated proviral DNA and in the United States remains the preferred test for early infant HIV diagnosis; approximately, 93% of HIV-infected infants will test positive by HIV DNA PCR assay by 2 weeks of age, and approximately 95% of infected infants will test positive by DNA PCR assay by 1 month of age.6 However, the HIV DNA PCR assay is less sensitive for identifying non-B-subtype virus and has been associated with false-negative tests in patients with non-B-subtype HIV infection.90
The HIV RNA PCR assay detects plasma viral RNA and can also be used for early infant diagnosis.90 The newer HIV RNA assay is as sensitive or more sensitive and as specific for detection of HIV subtype B compared to HIV DNA PCR.93 However, a false-negative result can occur in neonates receiving ARV prophylaxis.6 HIV RNA PCR may be more sensitive than HIV DNA PCR test for detection of non-B-subtype virus.94 Therefore, it is prudent to use HIV RNA PCR for diagnosis of infants born to women known or suspected to have non-B-subtype HIV infection.90
HIV DNA or RNA PCR testing is recommended at 14–21 days of age, and if test results are negative, repeat testing should be performed at 1–2 months of age and again at 4–6 months of age.6,90 Virologic testing is recommended at birth by some experts to diagnose in utero infection if mothers did not receive ART or prophylaxis during pregnancy or in other high-risk scenarios, but a cord blood specimen should not be used because of possible contamination with maternal blood. It is assumed that children who have a positive HIV PCR result within the first 48 hours after birth were infected in utero, whereas those who are infected during the intrapartum period might become positive 2 to 6 weeks after birth.38 An infant is diagnosed with HIV infection if 2 separate blood samples test positive for HIV DNA or RNA PCR.90
HIV isolation by culture is not recommended for routine diagnosis because culture is less sensitive, is more expensive, and needs a specialized laboratory, and results are not available for up to 28 days. Use of HIV-1 p24 antigen detection is not recommended for diagnosis of infant HIV-1 because of its poor sensitivity compared to HIV DNA PCR or culture.6
Hypergammaglobulinemia is a nonspecific but early finding of HIV infection. CD4 counts must be interpreted within the bounds of the age-dependent normal range, and changes in counts may result in a decrease in the normal ratio of more than 1.0 of CD4 to CD8 T-lymphocyte count.6
HIV infection can be presumptively excluded in non-breast-feeding HIV-exposed children younger than 18 months of age if there are (1) 2 negative HIV DNA or RNA PCR test results from separate specimens, both of which were obtained at greater than 2 weeks of age and 1 of which was obtained at greater than 4 weeks of age; or (2) 1 negative HIV RNA or DNA PCR test result from a specimen obtained at greater than 8 weeks of age; or (3) 1 negative HIV antibody test obtained at greater than 6 months of age; and no other laboratory (eg, no subsequent positive PCR test results if performed) or clinical (eg, no AIDS-defining illness) evidence of HIV infection.6,90
HIV infection can be definitively excluded in non-breast-feeding HIV-exposed children younger than 18 months of age if there are (1) 2 negative HIV DNA or RNA PCR test results from separate specimens, both of which were obtained at greater than 1 month of age and 1 of which was obtained at greater than 4 months of age; (2) 2 negative HIV antibody tests from separate specimens, both of which were obtained at greater than 6 months of age; and no other laboratory (eg, no subsequent positive PCR test results if performed) or clinical (eg, no AIDS-defining illness) evidence of HIV infection.6,90
In children with 2 negative HIV DNA PCR test results, many physicians confirm the absence of HIV infection by documenting a negative HIV antibody test result at 12 to 18 months of age (“seroreversion”).90 A non-breast-fed infant is considered HIV negative if 2 antibody test samples drawn at least 1 month apart and both obtained after 6 months of age are negative.6 If HIV antibody testing is performed at 12 months of age in an HIV-exposed infant not known to be infected, and if the infant is still antibody positive, repeat testing at 18 months of age is recommended. Detection of HIV antibody in a child older than 18 months of age is diagnostic of HIV infection. Documentation of seroreversion may be more important when non-subtype-B HIV is possible or present.
MANAGEMENT
Early Antiretroviral Therapy
If infection is confirmed, the infant should be promptly referred to a pediatric HIV specialist for consideration of HAART and care.95–99 Combination ART for HIV-infected infants and children is associated with improvement in growth and development; reduction in the risk of OIs, HIV encephalopathy, and other complications; and improvements of virologic and immunologic parameters. In the United States and other developed nations, use of HAART has resulted in dramatic reductions in mortality and morbidity of over 80%–90% in children.5,83,99 HAART has evolved from simple nucleoside reverse transcriptase inhibitor (NRTI) regimens of the 1980s and early 1990s to current complex regimens of NRTI in combination with protease inhibitors (PIs) or nonnucleoside reverse transcriptase inhibitors (NNRTIs).
Most HIV-infected children need HAART based on age, clinical, immunologic, and virologic criteria.96 The consideration of early treatment of asymptomatic infants is based on the rationale that infants are at highest risk of rapid disease progression to AIDS or death, even when immune degradation and virus replication are moderately well contained.98 Recent studies indicated that early initiation of combination ART within the first 3 months of life reduces morbidity and mortality compared with initiating therapy when symptomatic or immune suppressed.6,98 Early treatment can result in complete cessation of viral replication and the preservation of normal immune function.
Treatment Guidelines
Panels of experts have developed guidelines for the use of ARV therapy in children (available on line at http://www.aidsinfo.nih.gov).96 Pediatric HIV experts agree that infected infants with clinical symptoms of HIV disease or with evidence of immune suppression should receive HAART. Recently, a South African clinical trial (Children with HIV Early Antiretroviral Therapy [CHER] study) found that initiation of therapy at less than 12 weeks of age in asymptomatic infants with normal immune function resulted in 76% reduction in mortality and 75% reduction in HIV disease progression compared to waiting to initiate treatment of such infants until they met standard criteria for initiation of therapy.98 Based on the results of the CHER study, current US guidelines recommend initiation of ART for all HIV-infected infants younger than 12 months regardless of clinical status, CD4 T-cell count, CD4 percentage, or HIV RNA level (Table 58-6).96
Table 58-6 Indications for Initiation of Pediatric Antiretroviral Therapya



