Herpes Simplex Virus
INTRODUCTION
Neonatal herpes simplex virus (HSV) infection refers to any HSV infection occurring in infants within the first 28 days of life. Most infants present with symptoms within the first 2–3 weeks of life, and some cases are recognized as late as 4–6 weeks of age (rare cases recognized up until 8 weeks of age). Both HSV-1 and HSV-2 cause serious infection in the neonate. If infection is unrecognized and untreated, 50% of infants with central nervous system (CNS) HSV disease and 85% of infants with disseminated HSV infection die by 1 year of age.1 Advances in the diagnosis and treatment of neonatal HSV infection since the mid-1980s have improved the outcomes of infected infants.2 Despite advances in care, there is no evidence that the incidence of infection has decreased. Delay in diagnosis persists, and some infants who survive infection suffer devastating long-term sequelae.2
EPIDEMIOLOGY
Neonatal HSV infection is an uncommon disease, but early recognition and treatment are crucial in preventing mortality and long-term morbidity. Most neonatal HSV infections are in infants born to mothers with genital HSV-1 or HSV-2 infection. The remaining cases occur in infants exposed shortly after birth to a family member or health care worker with mouth or skin HSV-1 lesions. Based on serologic data from the National Health and Nutrition Examination Survey (NHANES), it is estimated that 22% of pregnant women in the United States are HSV-2 positive, 63% are HSV-1 positive, and 13% are seropositive for both viruses.3 Non-Hispanic white women were more likely to be seronegative for HSV compared to other racial and ethnic groups. HSV-2 causes most cases of genital herpes in the United States; however, genital infections with HSV-1 are increasing, and recent studies suggested that HSV-1 has now surpassed HSV-2 as a cause of genital herpes in college-age individuals.4 Women infected with HSV-2 are more likely to have recurrent symptomatic and asymptomatic shedding of virus compared to those with HSV-1.5
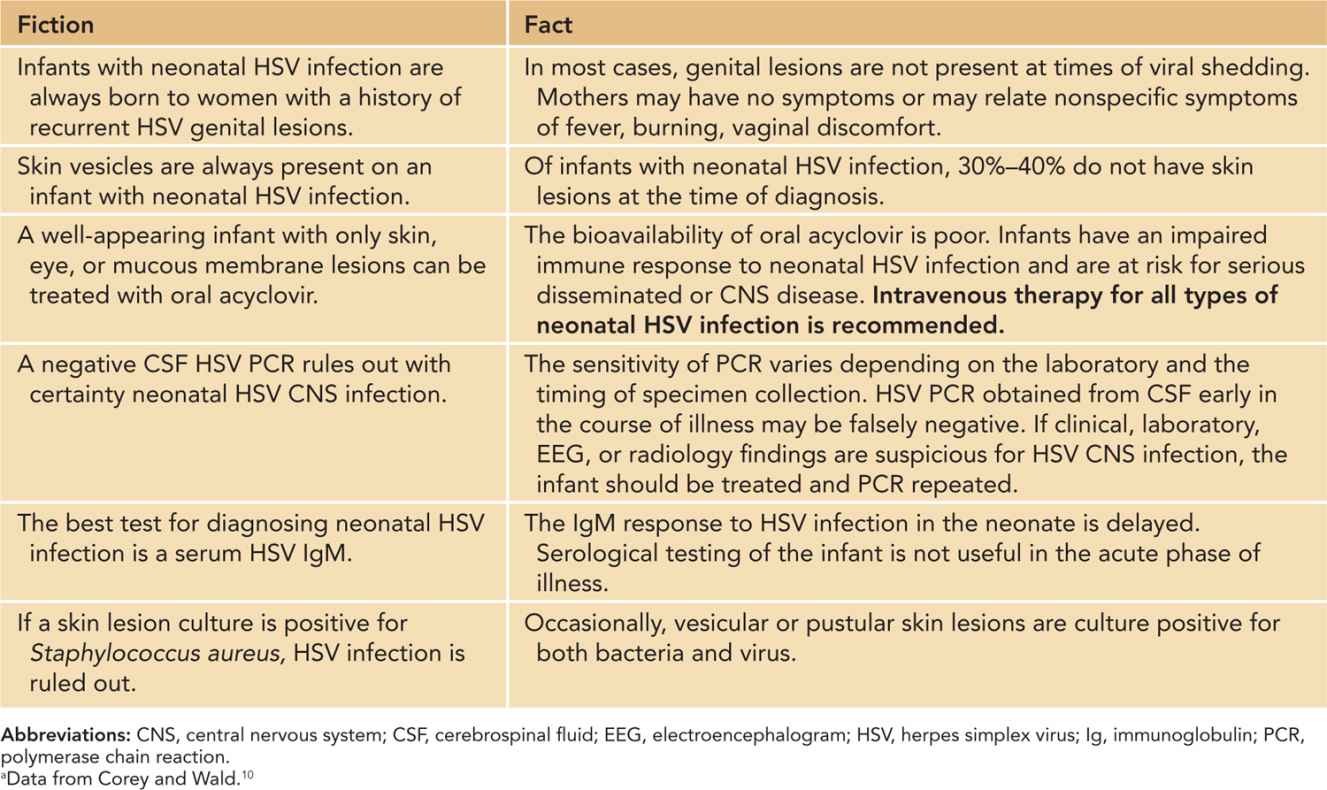
Most persons (70%–80%) with genital HSV have no symptoms; therefore, a history of no lesions during pregnancy does not rule out risk for neonatal HSV infection (Table 55-1). Some women with primary infection or recurrent disease have only nonspecific symptoms, such as fever or genital burning with urination that is mistaken for a urinary tract infection. Recurrence of genital herpes during pregnancy is common. Asymptomatic reactivation of genital HSV associated with short episodes of viral shedding and rapid clearance of virus likely occurs more frequently than previously thought.6
Table 55-1 Fiction and Fact Regarding Neonatal Herpes Simplex Virus (HSV) Infectiona
There are approximately 1500 cases of neonatal HSV infection in the United States each year.7,8 The exact number of cases is unknown because reporting of neonatal HSV infection to health departments is not required in most states.9 The rate of neonatal HSV infection in the United States varies depending on geographic area, study population, and study methodology.10 Studies utilizing state surveillance systems or hospital discharge data showed incidence rates of infection that range between 6 and 13 cases per 100,000 births.10–14 Hospital International Classification of Diseases, Ninth Revision (ICD-9) coding appears to be reasonably sensitive but not specific in identifying infants discharged with HSV infection.15 Use of ICD-9 codes in conjunction with chart review and confirmatory laboratory testing found the incidence of confirmed or probable neonatal HSV infection in 2 managed care organizations was 12.9/100,000 live births.15 One study utilizing ICD-9 coding found a substantially higher rate of infection in a large managed care population.16
There are several factors that may explain a difference in incidence rates. One important factor is that there are currently no ICD-9 codes specific for neonatal HSV infection. Investigators who utilize ICD-9 coding choose codes that, based on their clinical experience, are likely to identify neonatal infection and vary with each study.16 Standardization of coding or development of codes specific to neonatal HSV infection will improve the validity of future analyses. Many experts argue that neonatal HSV infection should be a reportable disease given its devastating effects in the most vulnerable of patients and their families and its comparable prevalence and increased morbidity in comparison to other reportable diseases.9
The only large prospective analysis8 of neonatal HSV infection was performed in Seattle, Washington, between 1982 and 1999. During the study period, the incidence of neonatal HSV infection was 31/100,000 births. The rate of transmission of infection was dependent on maternal serostatus. Infants born to mothers who were seronegative for both HSV-1 and HSV-2 during pregnancy were at highest risk (54/100,000 births) of infection, and those born to mothers who were seropositive for both viruses were at lowest risk (12/100,000 births).
Nonimmune pregnant women have an approximately 2% risk for developing a primary HSV infection during pregnancy.17 The most significant risk factor for neonatal HSV infection is maternal primary HSV infection around the time of delivery.17 As is typical with most primary genital HSV infections, the mother often has no symptoms but sheds significant amounts of virus from her cervix. The diagnosis of primary HSV infection in pregnancy cannot be based on clinical signs alone. A first HSV lesion may be reactivation of a previously asymptomatic infection. The diagnosis of primary HSV requires laboratory confirmation of HSV shedding (by viral culture or polymerase chain reaction [PCR]) plus either a negative antibody test with onset of symptoms or evidence of a change in serologic status over several weeks. An infant born to a mother with primary infection at the time of delivery has the highest (about 50%) risk of developing HSV infection.8,17 The reasons for higher risk in this scenario include exposure of the infant to the larger amounts of virus shed during a primary infection and little or no exposure to HSV type-specific neutralizing antibody.
Infants born to mothers with new infections that are first episodes but nonprimary appear to be at lower risk. An example of this situation would be an infant born to a mother who was previously seropositive for HSV-1 but then develops a first episode of genital HSV-2 around the time of delivery. In cases such as this, transmission rates are estimated to be 25%–30% or higher.
The lowest risk of neonatal acquisition occurs when the mother has a known history of genital HSV-1 or HSV-2 prior to or earlier in pregnancy and then is found to reactivate and shed the same type virus around the time of delivery.18 The estimated attack rate for neonatal herpes among these infants is less than 5%.
Other factors that increase the risk of neonatal infection if a mom is shedding HSV include use of fetal scalp electrode,19 prolonged rupture of membranes (>6 hours),20 known viral shedding at the time of delivery, cervical shedding, vaginal birth, and prematurity21 (Table 55-2). If maternal HSV lesions are noted at the time of labor, delivery of the infant by cesarean section is protective; however, neonatal HSV infection after cesarean section does occur.8
Table 55-2 Risk Factors for Neonatal Herpes Simplex Virus (HSV) Infection
1. Primary maternal HSV-1 or HSV-2 genital infection during pregnancy
2. Premature infant
3. Prolonged rupture of membranes
4. Vaginal delivery
5. Known maternal viral shedding, particularly cervical shedding
6. Use of fetal scalp monitors
7. Infant contact with caregiver with active HSV-1 skin or mouth lesions
Neonatal HSV infection is often diagnosed after an initially well-appearing infant has been discharged home from the nursery. One recent retrospective study found the prevalence of neonatal HSV infection in febrile infants admitted to the hospital from the emergency department similar to that of bacterial meningitis (0.2% vs 0.4%, respectively) but lower than that of serious bacterial infections (4.6%).22
PATHOPHYSIOLOGY
Etiology
Etiologically, HSV-1 and HSV-2 are enveloped, double-stranded DNA viruses in the family of Herpesviridae, subfamily alphaherpesvirinae.23 Typically, HSV-1 causes infection in the upper part of the body, including HSV gingivostomatitis, recurrent HSV oral lesions (cold sores), HSV keratitis, or herpetic whitlow. HSV-2 classically causes infection in the genital area. However, either virus can cause oral or genital infection.
Infection occurs when virus penetrates abraded skin or mucosa, replicates in the epidermis and dermis, and infects peripheral sensory nerve endings. HSV-1 and HSV-2 have the ability to establish latency and persist in sensory ganglion neurons for a patient’s entire lifetime.23 With infection of the oral mucosa, the site of viral latency is the trigeminal ganglia, and after genital infection, the site of latency is the sacral ganglia. The virus remains in a latent state with intermittent episodes of reactivation, causing virus excretion at mucosal or other sites. When reactivation is triggered, virus is transported back down axons to mucocutaneous sites, where replication and shedding of infectious HSV occurs. When epithelial cells are infected and destroyed, lesions are visible. However, excretion of infectious virus is often asymptomatic. As a result, infants with neonatal HSV infection are frequently born to mothers with no history of genital lesions at the time of delivery.
A number of HSV glycoproteins are required for infectivity and are targets of neutralizing antibodies. The amino acid sequence of 1 of these glycoproteins, glycoprotein G (gG) is varied enough that the antibody response to the molecule is different for HSV-1 (gG1) and HSV-2 (gG2).24 Because there is minimal cross-reactivity in antibody response to the gG for each virus, serological methods have been developed that are used to differentiate infection with HSV-1 or HSV-2.25 Use of type-specific testing has enhanced our understanding of the epidemiology and clinical presentation of each of these viruses.
Transmission
Infants may become infected with HSV in utero, intra partum (most cases), and postnatally. In utero infection results from transplacental transmission of HSV or ascending infection from the cervix26,27 and is considered rare, accounting for fewer than 5% of cases.28 In instances of presumed transplacental infection, necrosis of the placenta has been documented, and viral inclusions have been identified in placental trophoblasts. Acquisition of infection in utero has caused spontaneous abortion of the fetus. In some cases, an infant is born with signs of congenital infection. HSV DNA has been detected in the amniotic fluid of women who later gave birth to healthy infants.29 Risk factors for in utero transmission of virus from mother to infant are unclear because both primary and recurrent maternal infection have resulted in infection in utero. Ascending infection from the cervix through either ruptured or apparently intact membranes causing chorioamnionitis is an alternative cause of in utero infection.30
Most neonatal HSV infections occur at the time of birth when an infant’s mucosa or abraded skin comes in contact with HSV-1- or HSV-2-infected maternal genital secretions. HSV-1 causes 27%–45% of cases of neonatal infection.2,8 Although genital shedding with HSV-2 occurs more frequently than shedding of HSV-1, transmission of HSV-1 to the infant appears to be more efficient.8 Presenting symptoms in the neonate infected with either virus are similar. According to 1 study, approximately 9% of infants have clinical findings of infection on the first day of life,2 and some of these may reflect in utero acquisition of virus. Most infants appear well at birth, and it is not until viral replication at the site of infection occurs that symptoms appear. Infants with disseminated disease or skin, eye, and mucous membrane (SEM) disease typically present earliest (10–12 days of age), and some may develop symptoms within the first week of life. Infants with HSV infection localized to the CNS present later, at about 2–3 weeks of age.2,31 In some with isolated CNS disease, symptoms are not recognized until 4–6 weeks (and rarely as late as 8 weeks) of age. The reasons for later presentation of isolated CNS disease are unclear, but most of these babies have transplacental HSV antibody. The pathogenesis of disease is possibly retrograde axonal transport of virus to the CNS, rather than hematogenous spread of virus to the brain, as is seen in CNS involvement with disseminated HSV infection.
Approximately 10% of infants acquire HSV infection postnatally.31–33 An increase in the proportion of neonatal infection caused by HSV-1 may be in some part because of acquisition of virus in the days following birth. Postnatal infection can result from contact of the infant with infected secretions from the mother’s mouth or lesions on the skin. Infants have developed infection after nursing from a breast with HSV lesions or after ritual circumcision.33–35 Family members or hospital personnel with orolabial or skin lesions may also transmit infection to the newborn.36 The seroprevalence of HSV-1 in the general population is approximately 58% (30% to 90% depending on age and race/ethnicity).37 Many individuals have asymptomatic viral shedding, and those with close contact can unwittingly expose newborns to the virus. In addition, parents or family members may not realize that “cold sores” are caused by HSV and therefore pose a risk of infection to the newborn.
Immune Response
Because of an immature neonatal immune system, infants exposed to HSV in utero or within the first month of life have unique risks for viral dissemination. Neonates develop severe HSV disease that is not seen in older infants and children; therefore, early diagnosis and treatment are crucial. The developmental limitations in the neonatal immune response to HSV have not been fully elucidated but likely include deficiencies in both the early nonspecific innate and later antigen-specific adaptive immune response to HSV. Deficiencies in the function of neonatal natural killer and dendritic cells and decreased interferon-α (INF-α) production probably contribute to poor initial control of HSV infection.38 A key component in the immune response to HSV infection is development of adaptive cellular immunity to the virus. Newborns with HSV infections have a delayed T-lymphocyte proliferative response, and most infants have no detectable T-lymphocyte responses to HSV when evaluated 2 to 4 weeks after the onset of clinical symptoms.39,40 The delayed T-lymphocyte response to viral antigens in infants whose initial disease is localized to the skin, eye, or mouth likely explains the higher risk of progression to more severe disease in untreated infants.
Infants who receive transplacentally acquired neutralizing antibodies from the mother have a lower attack rate if exposed to virus.18 Infants who lack neutralizing antibody are more likely to have early onset or disseminated disease.39 The presence of passive antibody in the newborn, however, is not totally protective, and infants who have antibody at the onset of clinical symptoms and localized disease can develop disseminated or CNS infection if they do not receive antiviral treatment. Immunoglobulin (Ig) M antibody to HSV may be detected in some children 2–4 weeks after onset of symptoms. In previous studies, IgM was more often positive in infants with CNS disease and HSV-2, compared to those infants with SEM disease and infection with HSV-1.39
Finally, infants with higher titers of antibodies that mediate antibody-dependent cellular cytotoxicity (ADCC) appear to be at lower risk for disseminated infection than those with lower titers of ADCC antibodies.41
CLINICAL FINDINGS
Clinical findings in intrapartum and postnatal neonatal HSV-1 and HSV-2 infection are classified into 3 categories: (1) SEM disease; (2) CNS infection (encephalitis); and (3) disseminated disease, with or without CNS involvement. These clinical categories are assigned based on information learned from prospective studies performed by the National Institute of Allergy and Infection Diseases (NIAID) Collaborative Antiviral Study Group (CASG) in the 1980s and 1990s.2 The prevalence of each clinical category is reported to occur with approximately equal frequency2 but may vary depending on the time period and population studied.42,43 In addition, utilization of PCR analysis has identified infants with CNS disease who would previously have been categorized as having only SEM infection.44 Prognosis and treatment differ depending on the pattern of infection. Intrauterine HSV infection is discussed as a separate category.
Skin, Eye, and Mucous Membrane Disease
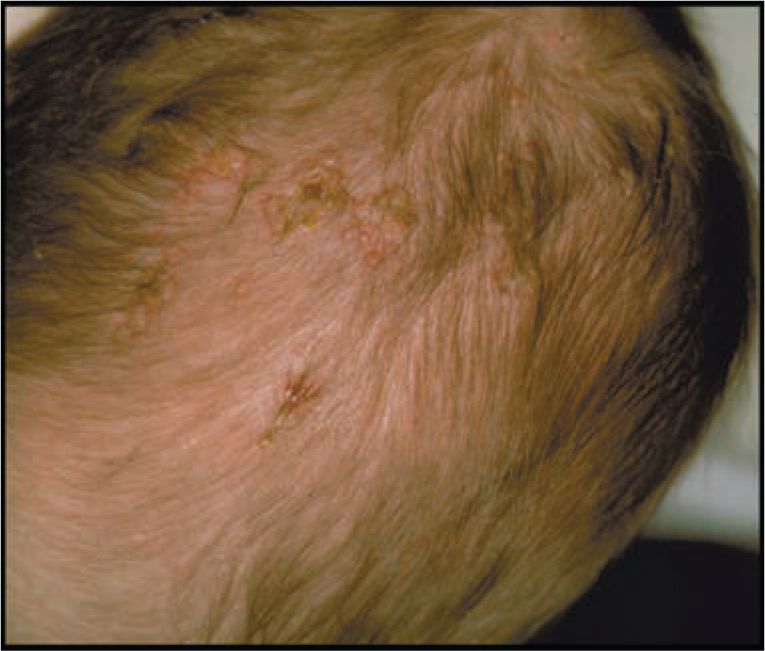
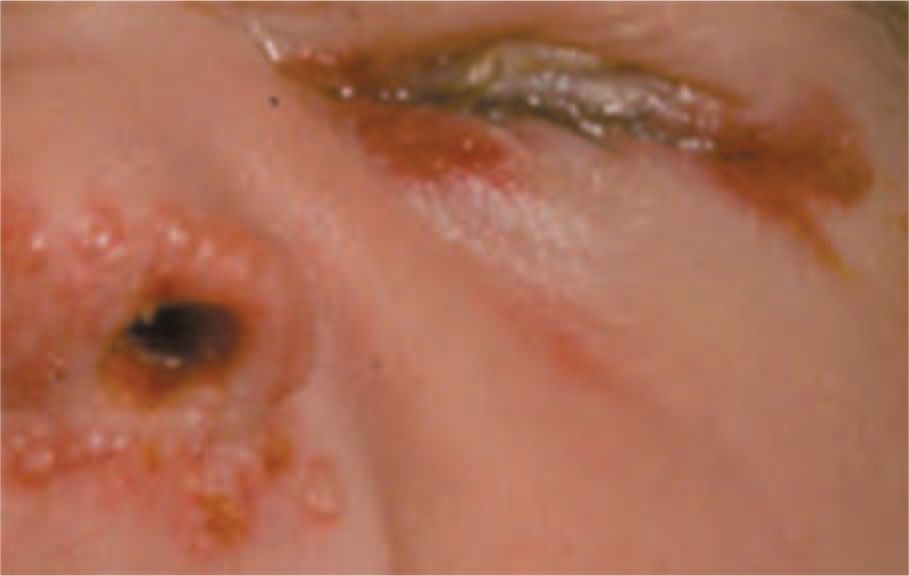
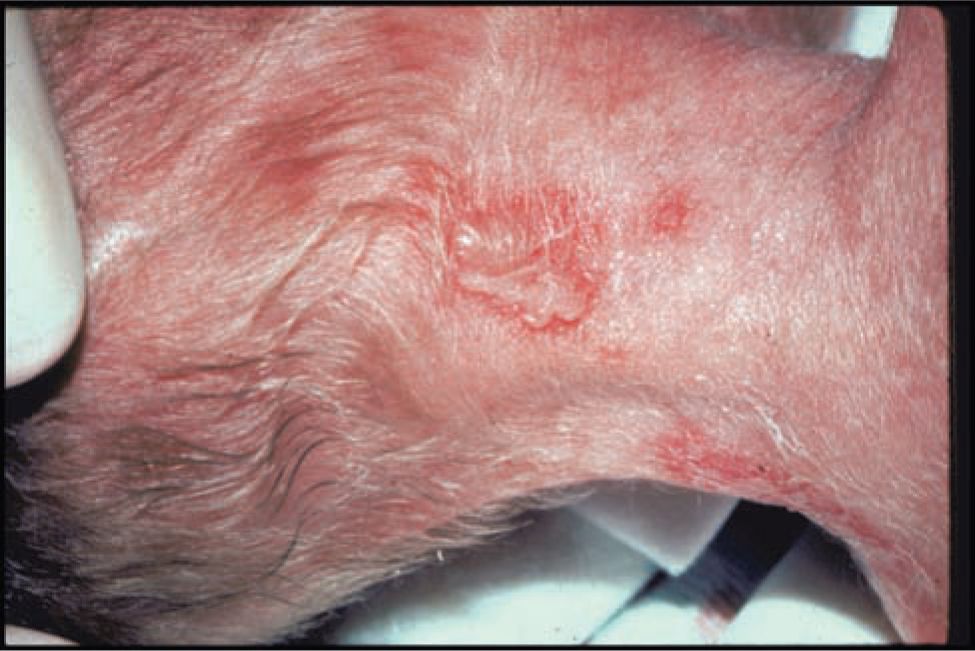
Infants with infection localized to the SEM typically present in the second week of life. Most infants with localized SEM disease are afebrile2,42 and appear well. Skin lesions are often identified on the presenting part or at sites of trauma (fetal scalp monitors). Skin lesions are small (usually < 0.5 cm) vesicles that appear individually or grouped on an erythematous base (Figures 55-1 and 55-2). Vesicles may coalesce into larger (>1 cm) bullous lesions (Figure 55-3). If infection is untreated, new lesions will appear in close proximity to initial lesions or distant to initial lesions, probably as a result of viremic spread. In some cases, primary vesicles crust to resemble staphylococcal or streptococcal impetigo. Approximately 17% of children with SEM disease do not have skin lesions initially and present with eye (Figure 55-2) or other mucous membrane involvement.2 HSV infection involving the eye causes keratoconjunctivitis and can progress to cause cataracts, corneal scarring, retinal necrosis, scarring, and detachment.45 HSV keratoconjunctivitis must always be considered in the infant with conjunctival injection, tearing, and discharge. Oral mucous membrane involvement with neonatal HSV can manifest as ulcerative lesions of the oral mucosa and tongue. In some cases, the presence of oral lesions is not noted, even with positive oral cultures. It is not clear whether this is because of poor physical examination techniques or whether oral secretions in the neonate are sometimes positive in the absence of obvious oral ulcers. Untreated neonatal SEM HSV is associated with high risk of progression to CNS or disseminated disease.46 Even after SEM disease is treated, infants have recurrent outbreaks of skin lesions throughout early childhood.47
FIGURE 55-1 Typical vesicular lesions on the top of the head of an infant with neonatal herpes simplex virus (HSV) infection. (Used with permission from Dr Ann Arvin.)
FIGURE 55-2 Typical vesicular lesions on the face of an infant with neonatal herpes simplex virus (HSV) infection. (Used with permission from Dr Ann Arvin.)
FIGURE 55-3 Larger coalesced herpes simplex virus (HSV) vesicular/bullous lesion, premature infant with neonatal HSV infection. (Used with permission from Dr Ann Arvin.)
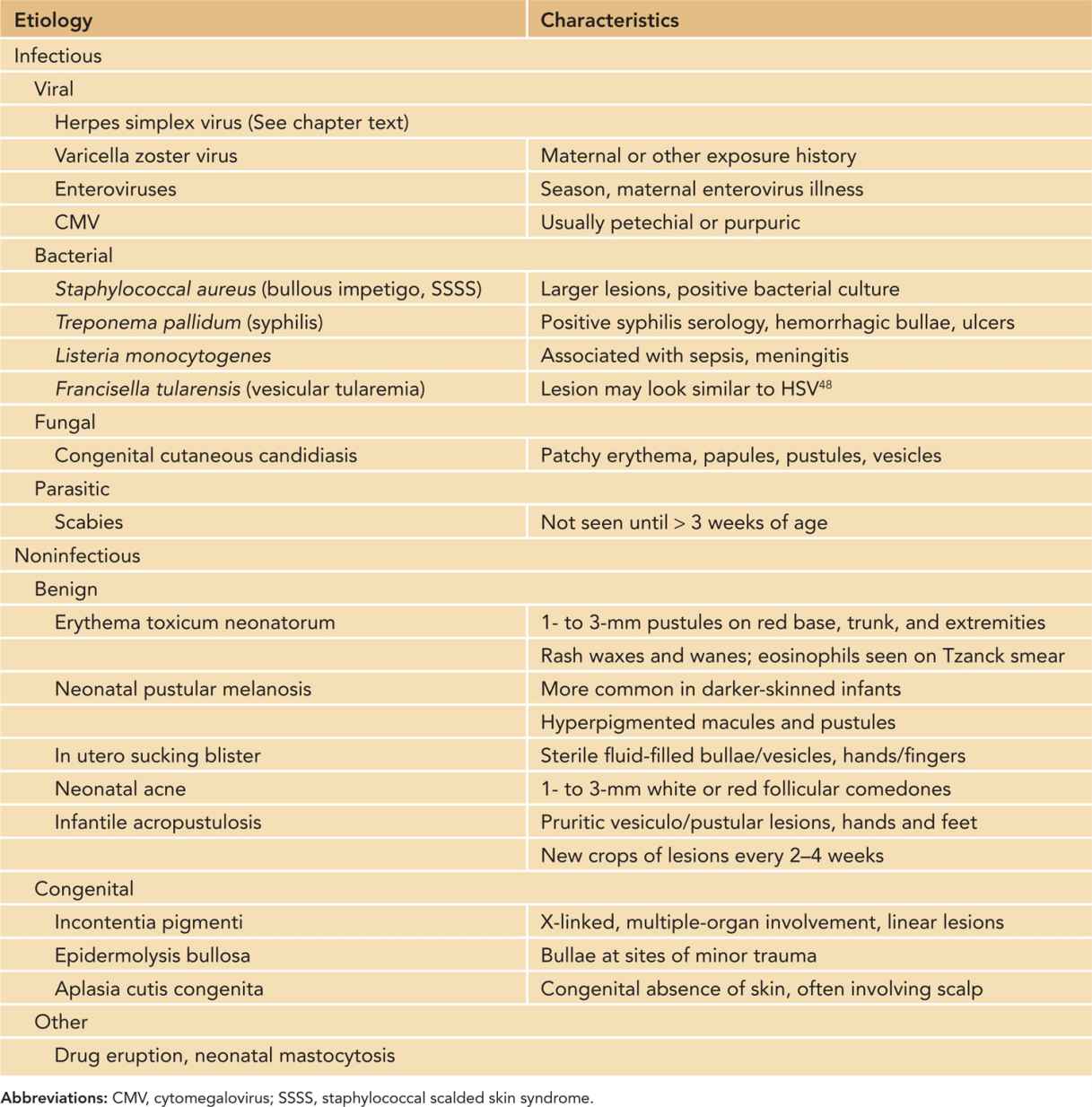
The differential diagnosis of neonatal HSV skin lesions includes both infectious and noninfectious causes of vesicular or pustular skin eruptions (Table 55-3). Of note, even if a lesion is found to be culture positive for a bacterial organism such as Staphylococcus aureus, testing for HSV should be performed if clinical suspicion is high because both pathogens may be present. Bacterial organisms such as Francisella tularensis48 and Listeria monocytogenes can cause vesicular or pustular lesions in an infant.
Table 55-3 Differential Diagnosis of Vesicular or Pustular Skin Lesions in the Newborn
Disseminated HSV Infection
Disseminated HSV infection carries the highest mortality of the different types of neonatal infection. Infants with disseminated HSV-1 or HSV-2 infection may become symptomatic within the first week of life, and almost all present by 3 weeks of age. Infants who are born prematurely are at higher risk for disseminated disease.43 Diagnosis of disseminated infection is often delayed because the signs and symptoms are frequently nonspecific and similar to those seen in babies with bacterial sepsis.42 Clinical features of disseminated neonatal HSV infection include temperature instability (fever or hypothermia), irritability, lethargy, shock, jaundice, bleeding, and respiratory distress. Progression of disease is rapid. Infection may involve any organ, including liver, lungs, gastrointestinal tract, adrenal glands, and brain. Fulminant hepatic failure is a complication of disseminated infection42 and likely is caused by high levels of circulating virus.49 Because CNS involvement is common in infants with disseminated HSV, seizures can occur. Laboratory abnormalities include elevated hepatic enzymes, thrombocytopenia, and a cerebrospinal fluid (CSF) pleocytosis, usually with a predominance of mononuclear cells, although polymorphonuclear neutrophil (PMN) cells may be present in the CSF.50 Skin lesions are present in about 60% of cases of disseminated infection. Lesions are often not noted at the beginning of illness but develop later, even after antiviral therapy has been initiated.2 One study found that a history of maternal fever was more common in mothers of infants with disseminated disease, compared to those with other categories of disease, and may be a surrogate marker for primary maternal infection.43
Differential Diagnosis
The differential diagnosis of disseminated neonatal HSV includes bacterial sepsis with typical neonatal bacterial pathogens and disseminated neonatal enterovirus infection.
Central Nervous System Infection
Infants with HSV-1 or HSV-2 encephalitis without disseminated disease typically present later, usually in the second or third week of life and occasionally as late as 4–6 weeks of age. In retrospect, history may reveal the presence of a skin lesion that pre-dated CNS symptoms. Clinical findings include fever, irritability, lethargy, poor feeding, seizures, a bulging fontanelle, or focal neurologic findings. Skin lesions may or may not be present at the time of diagnosis. In contrast to HSV encephalitis in adults, which is often localized to the temporal lobes, CNS-associated HSV infections in the neonate often involve multiple foci in the brain (Figure 55-4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree