Hemodynamics
Nick Evans
TRANSITIONAL CIRCULATION
An understanding of neonatal circulatory pathology is intrinsically linked to an understanding of circulatory adaptation to extrauterine life (see Chapter 43). The right-sided dominance of the fetal circulation, with blood shunting from right to left to bypass the lungs through the ductus and foramen ovale, has to change rapidly to the left-sided dominance of the extrauterine circulation with the whole cardiac output flowing through the lungs. In the fetal circulation, better oxygenated blood is returned from the placenta to the fetus via the umbilical vein. This blood is streamed by the ductus venosus across the right atrium, through the foramen ovale, and into the left atrium, facilitating delivery of the best oxygenated fetal blood to the brain and upper body. Blood returning via the vena cavae is streamed through the right side of the heart and into the descending aorta via the ductus arteriosus. This blood preferentially streams through the ductus because of arteriolar constriction and the high vascular resistance in the fetal lungs. The right-to-left ductal blood flow supplies the lower part of the body and also returns blood to the placenta via the umbilical arteries, which arise from the iliac arteries.
At birth, this blood flow pattern changes quickly. The lungs expand with the first breaths, the pulmonary arterioles dilate, right heart pressures fall, and blood pours into the pulmonary circulation to collect oxygen. The removal of the low-resistance placenta from the systemic circulation increases resistance and pressure on the left side of the circulation, while the pulmonary blood flow increases the left heart preload. The result is a dramatic increase in the workload of the left heart. The muscle in the wall of the ductus arteriosus constricts in response to rising oxygen levels, closing functionally within the first 24 hours after birth and structurally after several days to become a fibrous band.
During the last trimester, much of the fetal cardiopulmonary development is in preparation for the changes that have to occur at birth. Babies born prematurely have exquisite circulatory vulnerability during this period of the transitional circulation. More mature babies are also vulnerable if born in a compromised condition or if they become unwell shortly after birth.
CLINICAL ASSESSMENT OF CIRCULATION
Poor color, increased heart rate, prolonged capillary refill, and low urinary output suggest circulatory compromise. Blood measurements such as low pH and rising lactate can supplement the clinical assessments. These indicators are useful in identifying the baby with severe circulatory compromise, but they have limited accuracy for babies with lesser degrees of compromise.2
 BLOOD PRESSURE
BLOOD PRESSURE
Blood pressure can be accurately measured and continuously monitored if there is intra-arterial vascular access. Strong data exists for the importance of a normal blood pressure range4 (Fig. 56-1), but controversy arises over what constitutes an adequate blood pressure. A common assumption is that blood pressure equals blood flow, which has resulted in a focus almost entirely on blood pressure for the diagnosis and treatment of circulatory problems. However, because pressure is the product of flow and resistance, changes in either can affect blood pressure.
 MEASUREMENT OF BLOOD FLOW
MEASUREMENT OF BLOOD FLOW
Accurate measurement of blood flow is much harder than measuring blood pressure, but it better predicts oxygen delivery. The 2 methods most widely used for both research and clinical blood flow estimates are Doppler echocardiography and near infrared spectroscopy.
Doppler Echocardiography
Blood flow is the product of mean velocity and vessel cross-sectional area. Blood velocity can be measured with Doppler (eFig. 56.1  ), and vessel size can be measured with ultrasound (eFig. 56.2
), and vessel size can be measured with ultrasound (eFig. 56.2  ), as long as the vessel is of a reasonable size (see Chapter 495). These measures allow estimates of ventricular outputs and flow in other major vessels, such as the superior vena cava. Doppler measures in the smaller vessels are usually limited to velocity, which may not accurately represent flow. The advantage of Doppler ultrasound is that it measures the hemodynamic variable that most closely represents oxygen delivery. The disadvantages are the need for special equipment and skilled personnel and the potential for significant intrinsic errors, particularly in vessel size measurement.11 Further, because of the complexities of the shunts in the transitional circulation, ventricular outputs may not represent systemic blood flow in newborns.12
), as long as the vessel is of a reasonable size (see Chapter 495). These measures allow estimates of ventricular outputs and flow in other major vessels, such as the superior vena cava. Doppler measures in the smaller vessels are usually limited to velocity, which may not accurately represent flow. The advantage of Doppler ultrasound is that it measures the hemodynamic variable that most closely represents oxygen delivery. The disadvantages are the need for special equipment and skilled personnel and the potential for significant intrinsic errors, particularly in vessel size measurement.11 Further, because of the complexities of the shunts in the transitional circulation, ventricular outputs may not represent systemic blood flow in newborns.12
Near Infrared Spectroscopy
Near infrared spectroscopy uses infrared light to measure oxygenated and deoxygenated hemoglobin and, from these measures, blood flow in the superficial tissues of the brain (and other organs). Organ blood flow is derived, using the Fick principle, from change in oxy-hemoglobin in response to an increased inspired oxygen. Indices of tissue oxygenation can be derived from the ratios between oxy-hemoglobin and deoxyhemoglobin. Although a useful research tool for understanding neonatal hemodynamics, near infrared spectroscopy has not been incorporated extensively into clinical practices.13
PRETERM TRANSITIONAL CIRCULATION
The preterm baby is essentially an exteriorized fetus whose cardiovascular system is adapted to the low resistance of the placental circulation. Therefore, preterm babies in the intrapartum period and the first 24 hours after birth are vulnerable to circulatory compromise.
In the early hours after birth, low blood pressure and low systemic blood flow (SBF) can decrease cerebral blood flow (CBF) due to impaired cerebral autoregulation resulting in pressure-passive cerebral blood flow. Low blood pressure, SBF and CBF have been associated with ultrasound evidence of brain injury and adverse neurodevelopmental outcomes.6-8,14-17 Therefore, the relationships between SBF and CBF are a central focus in management of the preterm infant. Near infrared spectroscopy studies suggest there is a subgroup of preterm babies in whom autoregulation of CBF is compromised and who are at high risk for ultrasound evidence of brain injury.18,19 It is unclear whether absent autoregulation is the primary problem or is secondary to another insult.
Recent Doppler echocardiographic studies have focused on transitional hemodynamics in the heart with central measures of SBF. However, these measures can be confounded by left-to-right shunts through a patent ductus arteriosus or across a patent foramen ovale even in the early postnatal hours.6,12,20 Doppler measurements of superior vena cava (SVC) flow can be used to study the natural history of preterm SBF (upper body and brain).6,21 Low SVC flow occurs within the first 12 hours after birth in about 35% of babies born before 30 weeks, and flow improves by 24 to 48 hours.6 The magnitude of the low flow state correlates with the risk of intraventricular hemorrhage occurring as or after flow improves.6 There is also an association between lower average SVC flow in the first 24 hours after birth and poor 3-year developmental outcome.17
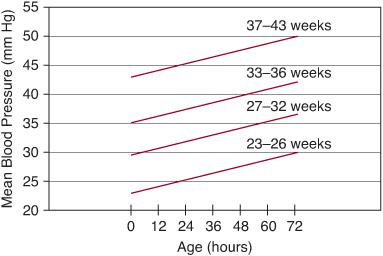
FIGURE 56-1. Gestational age–dependent and postnatal age–dependent nomogram for normal blood pressure. The lines represent the 10th centile for mean blood pressure for each gestational group.
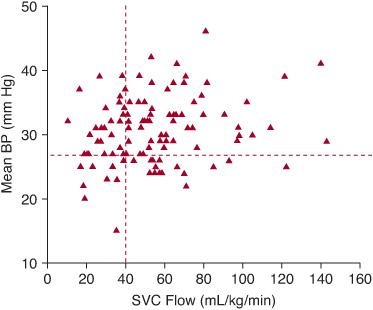
The causes of this low flow state are not known, but immaturity (lower gestational age) is the main risk factor. The preterm myocardium has a limited ability to respond to an increase in afterload,22,23 and the transition from the low-resistance intrauterine state to the high-resistance ex utero state may contribute to low SBF. The reduced capacity of the myocardium is further impacted by the requirement for positive pressure ventilation in many preterm infants, which is also known to compromise cardiac output.6 At the time of low SVC blood flow, babies are also more likely to have a larger patent ductus arteriosus (shunting blood out of the systemic circulation). Of clinical importance, there is only a weak relationship between blood pressure and SBF, whether measured by SVC flow or ventricular outputs.6-8 (Fig. 56-2), which indicates that peripheral vascular resistance must also decrease somewhat as flow increases, further compromising CBF.
This complex relationship with reduced myocardial capacity, low blood pressure and lost autoregulation that occurs in the preterm infant limits therapeutic options. Most practitioners attempt to maintain the blood pressure above an arbitrary level. Further therapeutic options will depend upon a better understanding of the factors that may improve autoregulation. Specific issues that may alter the preterm infant’s compensatory mechanisms are discussed below.
 HYPOVOLEMIA
HYPOVOLEMIA
Hypovolemia is an uncommon cause of circulatory compromise in preterm and term infants. There is often no relationship between blood volume and blood pressure. Occasionally, hypovolemia results from acute fetal blood loss. Usually, it is associated with intrapartum blood loss or fetoplacental transfusion (eg, after early clamping of a nuchal cord). These babies usually have classic features of circulatory compromise with pallor, tachycardia, hypotension, and a typical appearance on echocardiogram of poorly filled ventricles.
 PATENT DUCTUS ARTERIOSUS
PATENT DUCTUS ARTERIOSUS
Patent ductus arteriosus during the first week of life is associated with both lower systolic and diastolic blood pressures, and hence lower mean blood pressure,26 because the duct exposes the systemic circulation to the lower resistance of the pulmonary circulation throughout the cardiac cycle. See Chapter 55 for further discussion.
 SEPSIS
SEPSIS
Neonatal sepsis commonly presents with signs of circulatory compromise. In early-onset sepsis, the problems are probably a combination of the transitional hemodynamics and pulmonary hypertension associated with pneumonia. Severe shock associated with late-onset sepsis is usually associated with vasodilation and normal or high SBF (see eFig. 56.3  ).
).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


