Hematopoietic Cell Transplantation
Ann E. Woolfrey, Paul A. Carpenter, and Jean E. Sanders
Hematopoietic cell transplantation (HCT) transfers a small number of hematopoietic stem cells (HSC) from a donor to a recipient, where they are able to differentiate and proliferate to restore a normal hematopoietic and immune system (see eFig. 133.1  ). HCT is performed in patients with life-threatening hematologic disorders or as a means to restore hematopoietic function following administration of otherwise lethal doses of chemotherapy or radiation for treatment of resistant malignancies. HCT also has the potential to cure disorders resulting from defects in the pluripotent progenitor cells or in a single hematopoietic lineage.
). HCT is performed in patients with life-threatening hematologic disorders or as a means to restore hematopoietic function following administration of otherwise lethal doses of chemotherapy or radiation for treatment of resistant malignancies. HCT also has the potential to cure disorders resulting from defects in the pluripotent progenitor cells or in a single hematopoietic lineage.
INDICATIONS FOR HEMATOPOETIC CELL TRANSPLANTION
HCT is most commonly used to treat aggressive hematopoietic malignancies that have not responded to conventional therapy (see eFig. 133.2  ). Acute lymphoblastic leukemia is the most common indication for HCT in children who fail to achieve remission following induction chemotherapy, or in those that relapse following chemotherapy. In general, 65% to 75% of patients in first remission, 40% to 50% of those in second remission, and 10% of those with more advanced disease will survive long term (see Chapter 449).1,2 In patients with acute myeloid leukemia, HCT is generally indicated at first remission if an HLA–identical sibling donor is available (see Chapter 450).3 Other hematologic malignancies, such as chronic myelogenous leukemia, juvenile chronic myeloid leukemia, myelodysplastic syndromes, and myeloproliferative syndromes, are considered appropriate candidates for HCT, and in certain instances, HCT is the only potential modality for cure.4,5
). Acute lymphoblastic leukemia is the most common indication for HCT in children who fail to achieve remission following induction chemotherapy, or in those that relapse following chemotherapy. In general, 65% to 75% of patients in first remission, 40% to 50% of those in second remission, and 10% of those with more advanced disease will survive long term (see Chapter 449).1,2 In patients with acute myeloid leukemia, HCT is generally indicated at first remission if an HLA–identical sibling donor is available (see Chapter 450).3 Other hematologic malignancies, such as chronic myelogenous leukemia, juvenile chronic myeloid leukemia, myelodysplastic syndromes, and myeloproliferative syndromes, are considered appropriate candidates for HCT, and in certain instances, HCT is the only potential modality for cure.4,5
Hematopoietic cell transplantation (HCT) is also used to treat some pediatric solid tumors. The hematopoietic cells generally come from the patient, although other donors have been used. The transplant typically is performed after chemotherapy, and sometimes surgery or radiation, has reduced the tumor burden sufficiently. Neuroblastoma is the most common solid tumor for which HCT is used (see Chapter 457). Patients with relapsed Hodgkins or nonHodgkins lymphoma also may benefit from HCT, which results in approximately 50% long-term survival when performed during second remission.7 HCT also has shown promise as a component of treatment for patients with relapsed Ewing sarcoma or medulloblastoma.8,9
Life-threatening disorders of bone marrow production or immunity also may be treated with HCT. Patients diagnosed with severe combined immunodeficiency syndrome should be given HCT as soon as a suitable donor is identified (see Chapter 1).
Patients diagnosed with bone marrow failure also are considered excellent candidates for HCT. HCT may be the only potential for curing congenital marrow failure syndromes, such as Fanconi anemia or congenital neutropenias,16 and has been studied for treatment of other blood disorders such as thalassemia and sickle cell anemia. Prognosis is most heavily influenced by the underlying disorder. In general, results using HLA-identical sibling donors have been encouraging, with greater than 90% of patients surviving and greater than 80% living without recurrent disease symptoms.17,18 Mortality caused by the transplant procedure, and not from disease relapse, termed transplant-related mortality, ranges from 15% to 40% for allogeneic HCT recipients compared with 5% to 10% for autologous HCT recipients. HLA-disparity between donor and recipient increases the risk of transplant-related mortality owing to the greater likelihood of developing GVHD and graft rejection.
CLASSIFICATION OF HEMATOPOIETIC CELL TRANSPLANTATION
The type of hematopoietic cell transplantation (HCT) procedure appropriate for a specific patient depends upon the patient’s diagnosis, disease stage, prior treatments, donor availability, age, and presence of comorbidities. HCT is categorized according to the source of stem cells, the type of donor, or the intensity of the preparative regimen.
 STEM CELL SOURCE
STEM CELL SOURCE
HSC capable of reconstituting hematopoiesis can be obtained from three different sources: bone marrow, peripheral blood and umbilical cord blood. These HSC products are characterized by distinct kinetics of engraftment and recovery of immune function after transplantation. In general, HSC products with equivalent HLA-matching may be used interchangeably, however the risks of developing infectious complications and graft-versus-host disease (GVHD) may differ for HSC from different sources.
Bone marrow was historically the most common source of stem cells for HCT but its use has lessened as other sources have become more available.
Growth factor–mobilized peripheral blood stem cells (PBSC) are now the predominant source of HSC for allogeneic HCT in adults and for autologous HCT.19 PBSC are collected from the peripheral vein of the donor by leukapheresis. 
Umbilical cord blood (UCB) contains HSC sufficient for reconstitution of hematopoiesis, and can be collected from the placenta and umbilical cord immediately after delivery of a baby. UCB banking has increased donor availability for patients with rare HLA haplotypes.24 T cells contained in UCB are immunologically naïve, which allows for less stringent HLA matching between donor and recipient. The number of hematopoietic stem cells contained in a typical UCB unit is several orders of magnitude lower compared with that of typical bone marrow or PBSC harvests. The smaller number of HSC may result in delayed engraftment, increase risk for graft rejection, and infection.25 Nonetheless, UCB transplantation remains an important option with an acceptable toxicity profile for patients who would otherwise not have a suitable stem cell donor.26
 DONOR TYPE
DONOR TYPE
Transplantation of hematopoietic stem cells (HSC) donated by the patient is termed autologous hematopoietic cell transplantation (HCT). The success of the autologous transplant procedures relies exclusively on the tumor-eradicating potential of the preparative regimen. Transplantation of autologous HSC provides a means to overcome the marrow toxicity, hence to deliver markedly higher doses of chemotherapy or radiation.
Transplantation of marrow or PBSC donated from identical (monozygotic) twins is termed syngeneic hematopoietic cell transplantation (HCT).
Transplantation of hematopoietic cells donated by another individual is termed allogeneic HCT. Allogeneic HCT requires availability of an HLA-compatible related or unrelated donor. Because of the inheritance pattern of HLA haplotypes, the likelihood of two siblings being genotypically HLA identical is 25%. Donor-recipient HLA genotypic identity is associated with the lowest risks for immunologically mediated complications such as graft rejection and GVHD.27,28 For approximately 70% of patients who do not have an HLA-identical sibling donor, a search for an unrelated donor can be considered. HCT from HLA-matched unrelated donors, however, has traditionally been associated with higher risks of transplant-related morbidity and mortality compared with HCT from HLA-identical related donors. Use of unrelated donors who are matched not only at the antigen level (HLA typing by serology) but also at the genetic level (HLA typing by molecular methods) can improve outcomes considerably, and for some diseases, survival of patients with unrelated grafts has approached that of HLA-identical sibling grafts.29
Another alternative source of HSC is a haploidentical relative, such as a parent, defined by the inheritance of one identical haplotype and mismatching of one or more HLA loci with the noninherited haplotype. Over the past decade, technological advances have improved the outcome for recipients of HLA-disparate grafts.30
 INTENSITY OF THE PREPARATIVE REGIMEN
INTENSITY OF THE PREPARATIVE REGIMEN
In preparation for hematopoietic cell transplantation (HCT), high-dose chemotherapy alone, or combined with irradiation therapy, are used for the dual purpose of (1) eradicating the underlying disease process and (2) inducing immunosuppression to prevent graft rejection, an immunologic host-versus-graft reaction after allogeneic HCT. High-dose chemoradiation is followed by intravenous infusion of the hematopoietic stem cells (HSC) that home to the bone marrow and reconstitute the ablated hematopoietic system of the patient.
Myeloablative preparative regimens ablate the hematopoietic system of the patient. Although the regimens used for autologous HCT typically consist of drugs that provide maximum tumor eradication with tolerable toxicity to the patient, regimens used for allogeneic HCT also must provide sufficient recipient immunosuppression to prevent graft rejection. Myeloablative preparative regimens are associated with substantial risks of transplant-related toxicity and mortality, particularly among older or medically unfit patients.
Nonmyeloablative preparative regimens for allogeneic HCT are mainly immunosuppressive and aimed at preventing graft rejection. The underlying malignancy is eliminated through immunologic graft-versus-tumor effects, provided the tumor expresses antigens that make it a target for immune attack. Compared with myelo-ablative allogeneic HCT, the extrahematopoietic toxicity from nonmyeloablative preparative regimens is considerably milder. Thus, patients who would otherwise not be eligible for HCT because of age or comorbidities can take advantage of this treatment.31
COMPLICATIONS OF HEMATOPOIETIC CELL TRANSPLANTATION
Transplant-related complications are those resulting from undergoing hematopoietic cell transplantation (HCT), not from the underlying disease process. Transplant-related complications include (1) regimen-related toxicity, (2) infections, and (3) complications associated with alloimmune T cells.
 NON-INFECTIOUS REGIMEN-RELATED TOXICITY
NON-INFECTIOUS REGIMEN-RELATED TOXICITY
Regimen-related toxicities typically occur within the first month after myeloablative conditioning, and include cytopenias and organ damage. Each of these manifests differently in the immediate transplant period versus long-term sequelae, which are discussed separately below. More intense conditioning regimens also are associated with greater risk for infection, which is also affected by the prolonged period of immune reconstitution following allogeneic grafts. The complications of allogeneic HCT that may occur irrespective of the intensity of the conditioning regimen include rejection, graft-versus-host disease (GVHD) and hemolysis.
The likelihood of developing transplant-related complications depends on patient age, the intensity of the preparative regimen, the type and stage of the underlying disease, and the presence of comorbidities.
High-dose cytotoxic chemotherapy with or without doses of total body irradiation (TBI) exceeding 6 Gy has the potential to cause regimen-related toxicity (RRT) in the skin, gastrointestinal tract, liver, bladder, lung, heart, kidney, and nervous system. RRT occurs predominantly within the first 3 to 4 weeks after conditioning and is more common after myeloablative than nonmyeloablative conditioning.32-37 RRT increases the risk for opportunistic infection.32-37 Opportunistic infection or GVHD as etiologies for organ dysfunction must strongly be considered in the differential before making a diagnosis of RRT.
Pancytopenia
Reconstitution of hematopoiesis occurs in an orderly pattern; in general, neutrophil recovery occurs first, followed by recovery of platelets and red blood cells. Hematopoietic reconstitution varies according to the type of HSC product, being earlier after peripheral blood stem cells (PBSC) grafts and later after umbilical cord blood (UCB) grafts, relative to marrow grafts. Transfusions of 1500 to 3000 cGy irradiated platelets and red blood cells usually are needed to support hematopoietic function until hematopoiesis recovers. Red blood cell transfusions generally are indicated when the hemoglobin falls below 8 g/dL. Platelet transfusions are indicated when the platelet count falls below 10,000 cells/μL to minimize the risk for spontaneous bleeding.33 Patients that have become alloimmunized to platelet antigens demonstrate poor response to platelet transfusions and may achieve higher platelet counts by limiting the number of donor exposures, depleting transfused platelets of leukocytes, controlling fever or disseminated intravascular coagulation, use of platelet products that are less than 48 hours old, or use of non-pooled (single-donor) platelets or HLA-matched platelets.34
Precautions should be taken in preparation of blood products for transfusion into HCT patients because passenger lymphocytes pose a risk for generating GVHD. Except for the stem cell graft, all other components should be irradiated at a dose of 1500 to 3000 cGy to eliminate contaminating lymphocytes. Depletion of leukocytes or use of blood components that test seronegative for CMV are equally effective for prevention of CMV transmission to CMV-seronegative recipients.35 Removal of white blood cells from platelet and red blood cell products also decreases the risk for alloimmunization of the patient.36
Skin and Mucositis
Generalized skin erythema is common after doses of TBI exceeding 12 Gy but is self-limiting and rarely associated with skin breakdown. Regimens that contain cytosine arabinoside (Ara-C), thiotepa, busulfan, etoposide, and carmustine (BCNU) may also cause erythema. Hyperpigmentation typically follows the inflammatory dermatitis with skin folds often being particularly noticeable. Skin biopsies during the first 3 weeks after transplant often show nonspecific inflammatory changes irrespective of cause, making them usually unhelpful in distinguishing between RRT, drug allergies or acute GVHD.
Most patients who receive intensive conditioning regimens develop mucositis.37,38 Symptoms include inflammation, desquamation, and edema of the oral and pharyngeal epithelial tissue that typically presents within the first several days after HCT and usually resolves by the third week after HCT. Damage to the mucosa of the lower GI tract results in secretory diarrhea, crampy abdominal pain, and anorexia, and facilitates translocation of intestinal bacteria with sepsis.39 Anorexia, nausea, or other intestinal symptoms that persist after day 21 are more likely to be caused by graft-versus-host disease (GVHD) or infection.
Mucositis is treated supportively with administration of hyperalimentation and intravenous fluids to provide calories and maintain water balance, and intravenous narcotics to control pain. Octreotide or loperamide may be used if diarrhea is severe.41 It is important to recognize that an iatrogenic narcotic bowel syndrome, characterized by abdominal pain and bowel dilatation, may be a side effect of efforts to control painful symptoms of mucositis or liver toxicity.42 Esophageal bleeding conditions are treated supportively with transfusions to maintain platelet counts at more than 60,000 per μL and optimal management of emesis.
Hepatic Sinusoidal Obstruction Syndrome (SOS)
Hepatic sinusoidal obstruction syndrome (previously called veno-occlusive disease) develops in 10% to 60% of patients and is a clinical diagnosis based on the triad of tender hepatomegaly, jaundice, and unexplained weight gain usually within 30 days after HCT and in the absence of other explanations for these symptoms and signs.43,44 Once SOS is established, mathematical models can be used to predict prognosis, based on rates of increase in serum bilirubin and weight within the first 2 weeks after transplantation. The treatment for the 70% to 85% of patients who are predicted to have a mild or moderate course is largely supportive, with attention to management of sodium and water balance to avoid fluid overload.
Lung
Pulmonary complications occur in 40% to 60% of patients after HCT.45 Noninfectious pulmonary problems that may occur within 30 days from the transplant include idiopathic pneumonia syndrome (IPS), pulmonary hemorrhage, pulmonary edema due to excessive sodium and fluid administration or associated with sinusoidal-obstruction syndrome (SOS), or acute cardiomyopathy induced by cyclophosphamide, and sepsis with adult respiratory distress syndrome (ARDS).46,47 Although the incidence of life-threatening pulmonary infections has decreased over the past decade because of the introduction of routine antimicrobial prophylaxis, pulmonary complications continue to be a leading cause of death.
Heart
Cardiac complications related to chemotherapy or radiation occur in 5% to 10% of patients after HCT but death from cardiac failure is uncommon.48
Acute Renal Failure
Acute renal failure (ARF), defined by doubling of baseline serum creatinine, occurs in 30% to 50% of all patients during the first 100 days after hematopoietic cell transplantation (HCT), and most often during the first 10 to 30 days.50-52 Nephrotoxic drugs such as cyclosporine, tacrolimus, all amphothericin products, and aminoglycosides frequently cause renal insufficiency. Thrombotic microangiopathy, endothelial damage caused by chemoradiotherapy, cyclosporine, or tacrolimus, occurs in 5% to 20% of patients, more frequently in allograft recipients.
Hypertension
Hypertension develops in approximately 60% of patients after HCT, more often in patients given cyclosporine for GVHD prophylaxis. Most patients respond to conventional antihypertensive therapy, such as a calcium-channel blocker, angiotensin-converting enzyme inhibitor, or beta-blocker. Correction of hypomagnesemia, which often confounds cyclosporine therapy, may improve control of hypertension.55
Hemorrhagic Cystitis
High-dose cyclophosphamide is commonly used for conditioning and one of its toxic metabolites, acrolein, accumulates in the urine and may cause a hemorrhagic chemical cystitis during the conditioning regimen or later after HCT.56,57 Viral infections, particularly adeno-virus and BK virus, also have been implicated in the development of hemorrhagic cystitis and the diagnosis is established by viral culture or PCR test of a urine sample.59
Central Nervous System
Noninfectious complications include, cerebrovascular events, and encephalopathies because of metabolic, toxic, and immune-mediated causes. Focal symptoms are more indicative of infectious or cerebrovascular mechanisms, whereas diffuse symptoms such as delirium or coma may have metabolic causes. Fever is not necessarily associated with central nervous system (CNS) infections. Infection should be considered as the cause of any neurologic symptom and should prompt evaluation, including obtaining CT or magnetic resonance imaging (MRI) scans of the head and a sample of cerebrospinal fluid for appropriate cultures, cytochemistry stains, and PCR tests should be obtained.60
Cyclosporine or tacrolimus and glucocoricoids can cause a range of neurotoxicities.62,63 Essential tremor develops in most patients. Seizures have been reported in up to 6% of patients and may present in association with headaches, tremor, or visual disturbances. Seizures should be managed with anticonvulsant therapy and cessation of the drug if possible, or if not, substitution of one agent for the other. 
INFECTION FOLLOWING HEMATOPOIETIC CELL TRANSPLANTATION
Prevention of infection is of vital importance to the success of HCT procedures. Hospitalized patients should be housed in single rooms that have positive-pressure air flow and ventilation systems with rapid air exchange and high-efficiency particulate air filtration.66,67 Strict visitation, hand-washing, and isolation policies should be instituted to prevent introduction or spread of communicable disease. A daily program of skin and oral care should include bathing all skin surfaces with mild soap, brushing teeth with a soft brush, frequent rinsing of the oral cavity with saline, and good perineal hygiene. The diet should exclude foods known to contain bacteria or fungi, and patients should avoid exposure to dried or fresh plants or flowers. Caregivers should be trained in the proper handling of central venous catheters.68
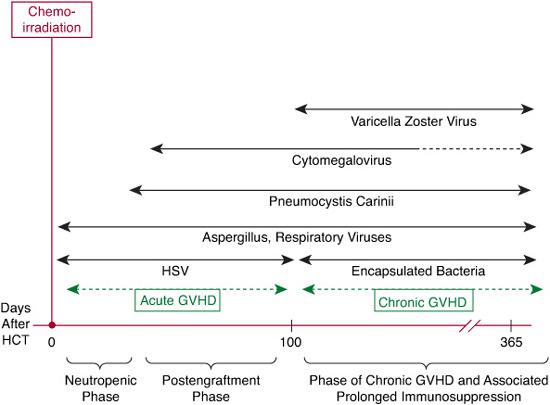
Immunologic reconstitution after HCT can broadly be categorized into 3 phases, which are characterized by a spectrum of opportunistic infections (Fig. 133-1). Prevention strategies for the most common infections through administration of appropriate antimicrobial agents are outlined in Table 133-1.
 BEFORE ENGRAFTMENT (<30 DAYS AFTER TRANSPLANT)
BEFORE ENGRAFTMENT (<30 DAYS AFTER TRANSPLANT)
This period is characterized by neutropenia and oral/gastrointestinal mucosal damage. The most common infections are bacterial (gram-positive and gram-negative) and fungal. Possible fungal infections that may occur during this period may be present with skin lesions (candida), sinus involvement (aspergillus and mucor), lung lesions (aspergillus), or hepatitis (candida). Herpes simplex virus is the most common viral infection in this period. Fever of unknown origin also occurs commonly during the neutropenic period.69 Prophylactic systemic antibiotics may be administered to reduce the risk of bacteremia during the neutropenic period, although improvement in survival has not been demonstrated.
 FOLLOWING ENGRAFTMENT (30–100 DAYS AFTER TRANSPLANT)
FOLLOWING ENGRAFTMENT (30–100 DAYS AFTER TRANSPLANT)
This period is characterized by skin and mucosal damage, and compromised cellular immunity related to GVHD and its treatment. Viral (cytomegalovirus [CMV]) and fungal (Aspergillus, Pneumocystis carinii) infections predominate during this period. Gram-negative bacteremias related to GVHD-associated mucosal damage and gram-positive infections due to indwelling catheters may also occur.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


