Heart Failure
Robert E. Shaddy and Chitra Ravishankar
Heart failure is a complex condition with many potential causes, but with the end result that the heart is unable to meet the metabolic demands of the body (including growth in children). Many believe that there must also be a component of systemic or pulmonary congestion. However, some patients with heart failure may have no significant congestion at rest, but only develop congestion with exertion or other forms of increased oxygen demand. Heart failure is generally precipitated by an insult to the cardiovascular system, either acquired or congenital. In adults, the most common insult is ischemic coronary artery disease with resultant left ventricular dysfunction. In children, heart failure is rarely ischemic, and the causes are quite varied and age dependent; refer to Chapters 483 and 484 for specific lesions associated with heart failure in children of different ages. Although infants with large left-to-right shunts and pulmonary overcirculation are commonly referred to as being in heart failure, their ventricular performance is usually normal, and their “heart failure” is usually a manifestation of pulmonary overcirculation with or without elevated ventricular filling pressures. They may have decreased systemic blood flow as well. Severe left-sided obstructive lesions (eg, hypoplastic left heart syndrome) often present with heart failure in the newborn period because the left ventricle cannot adequately eject blood to the systemic circulation. Both of these groups of lesions are generally managed by surgery or transcatheter intervention, but symptomatic therapy is often needed prior to surgical correction
Once the body has sensed a low cardiac output, a complex neurohormonal cascade is activated that includes the renin-angiotensinaldosterone system (RAAS) and the sympathetic nervous system (Fig. 497-1). This adaptive process causes fluid retention and stimulates release of vasopressive neurohormones such as norepinephrine in order to maintain or increase circulating blood volume and blood pressure. However, this cascade soon becomes maladaptive because it increases preload and afterload in an already overstressed system. Cellular responses are also initiated. For example, the augmentation of beta-adrenergic activity increases intracellular calcium which in turn increases myocardial inotropy and chronotropy. These adaptive mechanisms also soon become maladaptive, as prolonged beta-adrenergic stimulation of the heart leads to apoptosis and fibrosis.
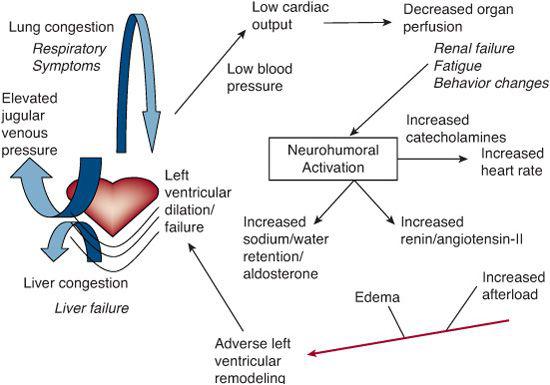
FIGURE 497-1. Schematic representation of heart failure syndrome. Regardless of the etiology, the pathogenesis of heart failure has similar mechanisms. A decrease in cardiac output results in decreased end organ perfusion and activation of a neurohormonal cascade. Stimulation of endogenous catecholamines and activation of renin angiotensin aldosterone system causes increasing heart rate, preload and afterload. These compensatory mechanisms increase myocardial oxygen consumption and eventually lead to reverse remodeling, ventricular dilatation, increased propensity for arrhythmias, and decreased coronary reserve.
The American Heart Association and the American College of Cardiology have divided heart failure into 4 stages: stage A: at risk for heart failure; stage B: structural heart disease without signs or symptoms; stage C: structural heart disease with previous or current symptoms; stage D: refractory heart disease requiring special interventions.1 The diagnosis and management of each of these stages in adults requires different modalities and approaches. Pediatric heart failure can also be considered in a similar light, although many of the proposed treatment strategies are different.2
CLINICAL PRESENTATION OF HEART FAILURE IN CHILDREN
Infants and young children present with poor feeding, failure to thrive, respiratory distress, diaphoresis, irritability or lethargy, and pallor. Older children frequently complain of poor appetite, abdominal pain, nausea, and vomiting, and occasionally present with chest pain, palpitations or syncope. Signs of heart failure include tachycardia, tachypnea, pallor, cool extremities, and peripheral edema. Young infants may have periorbital or facial edema. Jugular venous distension can be noted in older children. Cardiac examination reveals a prominent precordium with displacement of the apical impulse, frequently distant heart tones, and a gallop rhythm. Rales, particularly at the bases, and wheezing are heard in older children with pulmonary edema. Breath sounds may be diminished because of collapse of the left lower lobe as a result of compression of the left main bronchus by a dilated left atrium or as a result of pleural effusions. Tender hepatomegaly and ascites are not uncommon.
Chest radiography usually reveals cardiomegaly, pulmonary venous congestion or frank pulmonary edema (Fig. 497-2), although children with acute myocarditis may not have cardiomegaly at initial presentation. In addition there may be pleural effusions and left lower lobe atelectasis.
An electrocardiogram (EKG) may be useful. Children with fulminant myocarditis frequently have an abnormal EKG with low voltages, global ST segment elevation, or a wide complex rhythm (Fig. 497-3). It can also be diagnostic in infants with anomalous left coronary artery from the pulmonary artery (ALCAPA) where an anterolateral infarction pattern is frequently seen. EKG may also reveal arrhythmias such as supraventricular tachycardia, ventricular arrhythmias, or rarely heart block.
Heart failure is a clinical diagnosis, but echocardiography provides valuable supportive information regarding cardiac function and the details of anatomy.
ACUTE DECOMPENSATED HEART FAILURE OR LOW CARDIAC OUTPUT SYNDROME (LCOS)
A majority of children with heart failure are de-compensated on initial presentation. Symptoms of decompensation include breathlessness with minimal activity, orthopnea (inability to lie flat and/or use of multiple pillows), feeling faint or lightheaded, and gastrointestinal symptoms such as poor appetite, abdominal discomfort, nausea, and vomiting. Abdominal pain and nausea are ominous symptoms and should prompt further evaluation. A variety of clinical and laboratory parameters may help estimate the adequacy of cardiac output and oxygen delivery in the child. Peripheral pulse volume, capillary refill, blood pressure, urine output, and blood gas analysis indirectly assess cardiac output and oxygen delivery. In children, cardiac output is rarely directly measured, and is usually estimated using surrogate markers of oxygen delivery such as mixed venous oxygen saturation and serum lactate concentration.
Mixed venous oxygen saturation directly correlates with systemic oxygen extraction and thus is an excellent index of the adequacy of systemic blood flow. In patients without an in-tracardiac left-to-right shunt, the pulmonary artery oxygen saturation is the true “mixed” venous oxygen saturation. However, it is not easily accessed in young infants and children and cannot be used in patients with left-to-right shunting. The saturation in the superior vena cava (SVC) is also well mixed and approximates mixed venous saturation, though it is usually slightly lower due to the greater oxygen extraction of the upper body compared to that of the lower body. It is a very good surrogate for the mixed venous saturation in small children or older children with left-to-right shunting. Because an elevated serum lactate level indicates anaerobic metabolism, it is another good index of the adequacy of oxygen delivery, Several studies have demonstrated the usefulness of serum lactate in predicting outcome in adults and children after cardiac surgery, trauma, and similar events.
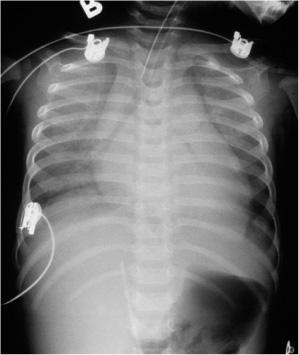
FIGURE 497-2. Chest radiograph of child with heart failure with cardiomegaly and pulmonary venous congestion and enlarged liver.
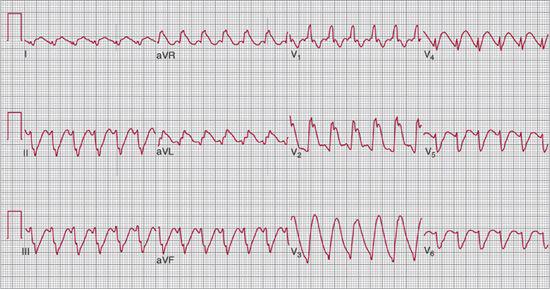
FIGURE 497-3. Electrocardiogram in child with heart failure due to myocarditis with wide complex rhythm.
Near infrared spectroscopy (NIRS) is a noninvasive technique which measures wavelength-specific absorption of light. It is used to measure oxyhemoglobin and deoxyhemoglobin in specific tissues. NIRS is now being used by many centers in the postoperative period to monitor cerebral and splanchnic oxygen saturations in order to assess the adequacy of oxygen delivery at the tissue level.
 BIOMARKERS OF HEART FAILURE
BIOMARKERS OF HEART FAILURE
Serum B-type natriuretic peptide (BNP) is a neurohormonal peptide secreted in response to ventricular dilatation. Normal serum BNP level is less than 100 pg/mL. Rising BNP levels are usually seen with worsening heart failure. In patients with heart failure, serum BNP level is usually significantly elevated, and is frequently greater than 1000 pg/mL. On the other hand, a decline in serum BNP level can indicate clinical improvement. Thus it is useful to follow serial serum BNP levels both while escalating therapy for worsening heart failure, and during weaning from inotropic support and afterload reduction.
 TREATMENT OF ACUTE DECOMPENSATED HEART FAILURE
TREATMENT OF ACUTE DECOMPENSATED HEART FAILURE
Factors that influence cardiac output such as preload, afterload, myocardial contractility, heart rate and rhythm must be assessed and manipulated.3
 PRELOAD VERSUS FLUID OVERLOAD
PRELOAD VERSUS FLUID OVERLOAD
Patients with acute decompensated heart failure and low cardiac output syndrome (LCOS) are usually fluid overloaded; thus fluid boluses should be avoided or small aliquots (5–10 mL/kg) should be used with careful monitoring of vital signs. Rapid infusion of large volume of fluid may suddenly distend the heart and cause bradycardia, hypotension, and cardiac arrest. Most of these patients require intravenous diuretics such as furosemide. In fact, many patients will have a remarkable clinical improvement with this simple therapy, and may not need additional pharmacologic support.
 PHARMACOLOGIC SUPPORT
PHARMACOLOGIC SUPPORT
Patients presenting with decompensated heart failure frequently require agents that provide inotropy and/or afterload reduction (Table 497-1). The treatment of choice for many centers is the use of inodilators (inotropes with afterload reducing properties) such as milrinone.4 It has replaced catecholamines such as dopamine, dobutamine, and epinephrine as first line of therapy in children with low cardiac output syndrome (LCOS). Milrinone is a nonglycoside, noncatecholamine inotropic agent with additional vasodilatory and lusitropic properties. It inhibits phosphodiesterase type III, increasing intracellular cyclic adenosine monophosphate (AMP) and intracellular calcium, thus enhancing myocardial contractility. It also enhances diastolic relaxation of the myocardium by increasing the rate of reuptake of calcium after systole. It may also act synergistically with a beta-adrenergic agonist such as dopamine and has fewer side effects. Phosphodiesterase type III inhibitors have been used extensively in adults and more recently introduced to pediatric practice. Milrinone lowers filling pressures, systemic and pulmonary artery pressures, and systemic and pulmonary vascular resistances, while improving cardiac index. Milrinone is usually initiated at 0.25 µ/kg/minute and can be titrated gradually to 1 µ/kg/minute based on clinical response.5 It is prudent to avoid a bolus of milrinone in patients with LCOS, who are either normotensive or hypotensive at presentation. Milrinone has also replaced amrinone because of the relatively high incidence of thrombocytopenia associated with the latter. Catecholamines, either endogenous (eg, dopamine or epinephrine) or synthetic (eg, dobutamine) act by stimulating myocardial surface beta-adrenergic receptors, leading to increased adenylate cyclase and intracellular cyclic adenosine monophosphate (cAMP).
Table 497-1. Class of Recommendations and Level of Evidence



