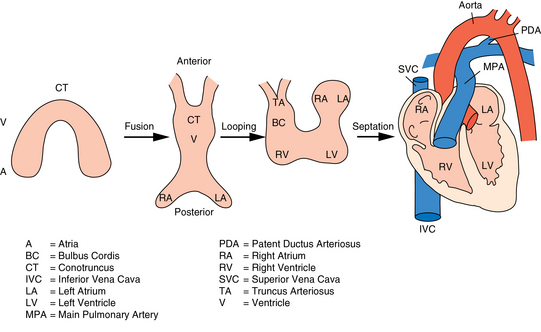
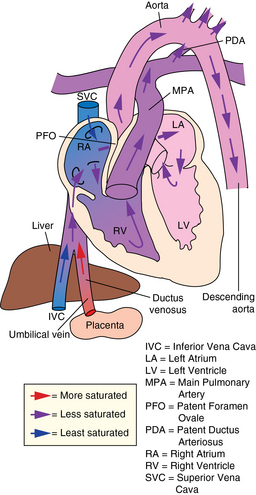
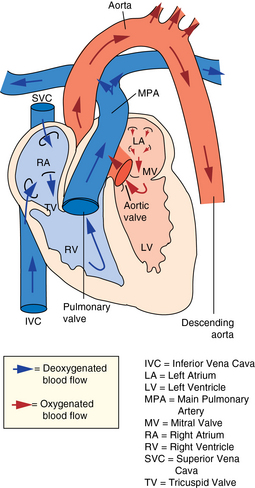
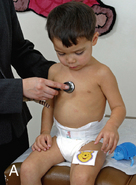
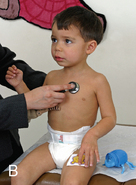
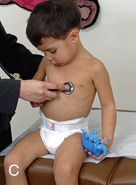
CHAPTER 7 The heart begins to form in the fetus by the end of the third week after conception. A crescent-shaped structure is formed that fuses at the midline to create a single linear heart tube (Figure 7-1). As the primitive heart tube elongates, it differentiates into the atria, ventricles, bulbus cordis, and truncus arteriosus. The conduction system also begins to form during this time and by day 23 after conception the heart begins to beat. Valve formation begins around the fourth to fifth week after conception, and the formation of the heart is complete by the eighth week after conception. Any early changes in this process caused by genetic, maternal, or external environmental factors can lead to structural malformations of the heart. During fetal life, the lung sacs are collapsed and blood is oxygenated through the placenta. Oxygenated blood travels from the placenta to the heart via the umbilical veins and ductus venosus to the inferior vena cava (IVC) and into the right atrium (RA). Blood then streams to the left atrium (LA) through a patent foramen ovale (PFO) and into the left ventricle (LV), which pumps it out the aorta (Figure 7-2). The less saturated venous blood traveling from the superior vena cava (SVC) and coronary sinus also flows to the right atrium, but is directed toward the right ventricle (RV) and pulmonary artery (PA). High pulmonary vascular resistance limits blood flow into the lungs, which are not yet involved in ventilation, and redirects it through the patent ductus arteriosus (PDA) to the descending aorta and lower body. With a baby’s first breaths, pulmonary vascular resistance falls, causing a dramatic increase in pulmonary blood flow. The ensuing increase in pulmonary venous return to the heart raises LA pressure, causing closure of the PFO. Arterial oxygen saturation increases as a result of improved oxygenation by the lungs. This higher saturation promotes functional closure of the PDA by 48 to 72 hours after birth, with complete anatomical closure occurring by 2 to 3 weeks of age.1 The heart is composed of four chambers. The upper chambers (atria) are low-pressure receiving chambers, and the lower chambers (ventricles) are high-pressure pumping chambers. The heart is further divided into right and left sides. The RA receives deoxygenated blood from the body, and the RV pumps it out the pulmonary artery to the lungs to become oxygenated. The LA receives oxygenated blood from the lungs, and the LV pumps it out the aorta to the body (Figure 7-3). The LV operates at a higher pressure than the RV. This normal circulation occurs in series, and there is no mixing of deoxygenated and oxygenated blood. There are four valves in the heart that regulate blood flow between the atria and ventricles. The atrioventricular (AV) valves regulate blood flow between the atria and ventricles, and the semilunar valves regulate blood flow between the ventricles and great vessels. The tricuspid valve on the right and the mitral valve on the left are the AV valves (see Figure 7-3). The pulmonic valve, located at the base of the pulmonary artery between the right ventricle and the pulmonary artery, and the aortic valve, located at the base of the aorta between the aorta and left ventricle, are the semilunar valves. Closure of these valves produces the heart sounds commonly referred to as “lub-dub” (S1, S2). In preterm infants, the ductus arteriosus may remain open for several weeks after birth causing hemodynamic instability. Medical intervention with a prostaglandin inhibitor, such as the drug indomethacin, or surgical ligation of the ductus is required to stabilize the infant.1 A careful and systematic history needs to be performed at each developmental stage to monitor the overall health status of infants and children, to identify those with cardiac symptoms, and to recognize signs of cardiac disease. The Information Gathering table presents the relevant age-related questions to ask about the cardiovascular system and questions related to maternal infections and maternal medical conditions that place infants at high risk for congenital heart disease (CHD). Information Gathering for Assessing the Cardiovascular System at Key Developmental Stages The cardiac examination must be systematic and tailored to the child’s developmental level (Figures 7-4 and 7-5). A complete assessment of the cardiac system must be done in order to make conclusions about the significance of any single abnormality. Cardiac findings should not be taken in isolation. It is important to consistently plot height, weight, and head circumference in infants and children up to age 2 years of age to evaluate whether the growth rate is proportional and to monitor for failure to thrive (FTT). For infants, any drop-off in weight percentiles as compared to length and head circumference values should raise the suspicion of CHD.1 Temperature, heart rate, and respiratory rate should be measured and assessed. Fever and respiratory distress both can elevate heart rate. Blood pressure should be measured whenever possible during physical examinations, but definitely once in infancy and then routinely after age 3.2 Coarctation of the aorta and systemic hypertension can go undetected if blood pressure measurements are omitted during well-child visits. If measured, blood pressure in infants should be taken in all four extremities or at a minimum in the right arm and in one leg to detect a coarctation of the aorta. In children, simultaneous palpation of the radial and femoral pulses is also important in assessing whether a coarctation may be present. Clubbing occurs when arterial desaturation has been present for at least 6 months or longer. The fingers and toes become red and shiny, and progress to wide, thick digits with eventual loss of the normal angle between the nails and the nail beds2 (Figure 7-6). It should be noted that infants born with cyanotic heart disease are being treated earlier than in the past, and this has resulted in a decreased incidence of clubbing in children and adolescents. Pulses need to be evaluated for their presence or absence, intensity, timing, symmetry, and whether the pulse is regular or irregular, weak or bounding. A comparison also should be made as to right and left symmetry and quality of pulses in the upper and lower extremities. A pulse that is absent or weaker in the lower extremities compared to the upper extremities is diagnostic of coarctation of the aorta. A strong pedal pulse is a good indication that there is no coarctation. Irregular pulses may be due to an arrhythmia. Weak and thready pulses may indicate poor perfusion or shock, whereas bounding pulses are usually noted with aortic run-off lesions such as a PDA, AV malformation, or aortic insufficiency.2 Peripheral perfusion is also important to assess, especially in infants. Normally, extremities should be warm to the touch and have a brisk capillary refill time (CRT) of less than 3 seconds—a quick measure of cardiac output. Older children with cardiac conditions may have weak distal pulses on one side or the other because of previous cardiac catheterizations or cardiac surgeries. The precordium should be palpated to determine the location of the point of maximal impulse, or PMI. The PMI is important in determining ventricular overload, cardiomegaly, and the presence or absence of thrills. Normally, the PMI is felt at the apex in the left midclavicular line, indicating LV dominance. However, it is normal for newborns and infants to have a greater RV impulse, with the PMI felt at the left lower sternal border (LLSB). A PMI that is diffuse and rises slowly is called a heave, and a PMI that is sharp and well localized is known as a tap.2 A thrill indicates turbulent blood flow and is never normal. It is felt as a vibratory sensation and should be examined not only on the precordium but also in the suprasternal notch and over the carotid arteries. Precordial thrills are best felt with the palm of the hand, whereas thrills in the suprasternal notch and over the carotid arteries are best felt with the fingertips.2 Auscultation of heart sounds in children should be done in a stepwise fashion and with both the diaphragm and the bell of a stethoscope to elicit both high (diaphragm) and low (bell) frequency sounds (Figure 7-7). Most children have a thin chest wall and heart sounds are louder than in adults. However, the faster heart rate can make it difficult to accurately distinguish the heart sounds from other adventitious sounds, particularly in early infancy. Thus it is recommended that the individual heart sounds be identified and analyzed before identifying murmurs. The first heart sound is called S1 and is created by the closure of the tricuspid and mitral valves. It is usually heard best at the LLSB or at the apex. A split S1 can be a normal but uncommon finding in children.2 The second heart sound is called S2 and is created by the closure of the aortic and pulmonic valves. It is usually heard best at the left upper sternal border (LUSB). Evaluation of S2 is critical in children because it provides important clues as to the presence of structural defects and to the pressures in the heart. S2 normally varies with respiration—split with inspiration and single or narrowly split in expiration. A fixed split, single S2, or loud S2 warrants further evaluation by a cardiologist. Abnormal splitting of the S2 may indicate increased pulmonary blood flow, pulmonary valve abnormality, or a cyanotic heart condition. A loud single S2 may indicate pulmonary hypertension or malposition of the great arteries.2 A fourth heart sound (S4 or gallop rhythm) is rare in infants and children. An S4 is an abnormal finding and suggests decreased ventricular compliance in conditions such as CHF.2 Ejection clicks are extra heart sounds that occur between S1 and S2. They are heard best at the upper sternal border and are usually associated with stenotic semilunar valves or dilated great arteries.2 Murmurs are produced when blood flows across an area that has a pressure difference and causes turbulence or disturbed flow. Murmurs should be assessed and evaluated according to their timing in the cardiac cycle, location, transmission, intensity, frequency, and quality.2 It is always important to note whether a murmur radiates to the lung fields, axillae, clavicles, or neck. A normal grading scale is used to describe a murmur’s intensity (Table 7-1). TABLE 7-1 Grading Scale for Cardiac Murmurs Data from Park M: Pediatric cardiology for practitioners, ed 5, St. Louis, 2008, Mosby.
Heart and vascular system
Embryological development
Anatomy and physiology
Anatomy of the postnatal heart
Physiological variations
System-specific history
Age-Group
Questions to Ask
Rationale
Prenatal, infancy, early childhood
Any family history of CHD/chromosomal abnormalities, sudden/premature death; maternal illness/infections (both chronic and during pregnancy); maternal medications/drug use
CHD caused by interaction between genetic and environmental factors (systemic lupus, rubella, diabetes mellitus, anticonvulsants, alcohol, etc.)
Apgar scores
Usually normal with CHD except for color if cyanotic
Any problems with poor feeding, sweating (especially on the forehead) during feeding, weight loss/gain, FTT, decreased activity level
Symptoms of CHD often occur with feeding because of increased oxygen consumption and need for greater cardiac output
Any pallor or blueness in color; any changes in color, especially when crying
Babies with cyanotic heart disease turn dark blue or ruddy in color when crying because of prolonged expiratory phase and resulting increase in right-to-left shunting. Hypercyanotic spells are often associated with extreme irritability and rapid, deep, and sometimes labored respirations.
Any pattern of rapid breathing; frequent respiratory infections
Left-to-right shunting lesions (VSD, AVSD, PDA) cause increased blood flow to the lungs, resulting in frequent respiratory infections, FTT, and decreased exercise tolerance.
Middle childhood and adolescence
Any inability to keep up with the activity level of peers, need for frequent periods of rest, anorexia, cough, wheezing, rales, chest pain, leg cramps, syncope, light-headedness, palpitations; any history of drug use; any family history of sudden death, syncope, or arrhythmias
CHD can decrease exercise intolerance. Left ventricular outflow obstructive lesions (AS, coarctation of the aorta) can cause CHF. Undetected coarctation of the aorta can cause leg cramps. Coronary artery abnormalities (including Kawasaki disease) and cocaine use can cause chest pain. Structural and dysrhythmic heart disease can first present as syncope. Some dysrhythmias are genetic in origin and run in families.
Any history of recent infections, prolonged fever or malaise, recent dental work
Untreated streptococcal infections, Kawasaki disease, and subacute endocarditis can result in CHF or acquired heart disease.
Physical assessment
Inspection
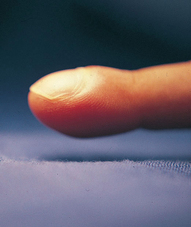
Clubbing
Palpation
Auscultation
Heart sounds
Murmurs
Grade
Sound
1
Barely audible and softer than usual heart sounds
2
Still soft, but about as loud as usual heart sounds
3
Louder than usual heart sounds, but without a thrill
4
Louder than usual heart sounds, and with a thrill
5
Can be heard with stethoscope barely on chest (rare)
6
Can be heard with stethoscope off chest, or with naked ear (extremely rare)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Heart and vascular system
Only gold members can continue reading. Log In or Register to continue