Gynecologic and Fertility Issues for Cancer Patients/Survivors
Sara Barton
Marc R. Laufer
Advances in diagnosis and therapies have greatly enhanced the life expectancy of children and adolescents with malignancies (1). Of the >12,000 children and adolescents diagnosed with cancer, long-term survival is expected for 80% (2). For example, children with acute lymphoblastic leukemia who did not receive radiation therapy and who are at least 10 years cancer free can expect a normal long-term survival (3). Information regarding cancer survivorship is available at the National Cancer Institute’s Surveillance, Epidemiology, and End Results Web site, which is updated on a regular basis (http://seer.cancer.gov/csr). In addition, the Children’s Oncology Group maintains an updated Web-based resource on survivorship care at http://www.survivorshipguidelines.org.
While the cancers themselves may have an impact on reproductive function, the treatment-specific therapies (surgery, chemotherapy, and/or radiation) may produce long-term adverse reproductive outcomes (4). Furthermore, pediatric cancers often require combined modality protocols, resulting in additive adverse effects on gonadal function (2) and the hypothalamic–pituitary axis. Although much of the available literature uses amenorrhea as a surrogate marker for infertility, the absence of menses is neither sensitive nor specific in identifying survivors at risk for early gonadal failure or infertility in the future. Studies have interchanged the terms gonadal failure, premature menopause, ovarian failure, and ovarian insufficiency (see Chapter 9). There is currently a trend to use the term ovarian insufficiency, but the reality is that there can be a changing or fluid state of ovarian function over time after exposure to chemotherapy and/or radiation therapy. For the purposes of this chapter, these terms collectively refer to loss of endocrine function of the ovary. Premature menopause is defined as menopause prior to age 40. Efforts to develop sensitive markers of ovarian reserve and to accurately describe the effects of cancer therapy on reproductive function in females are ongoing (5).
Pubertal Development, Menstrual Function, and Fertility
Results from the Childhood Cancer Survivor Study indicate that acute ovarian failure and amenorrhea occurred in 6.3% of childhood cancer patients and was associated with high-dose radiation exposure of the ovaries, treatment with procarbazine or with cyclophosphamide and treatment between 13 and 20 years old (as opposed to younger ages) (6). Premature menopause in this population was increased 10-fold, occurring in 8% of survivors versus 0.8% of sibling controls. Premature menopause was associated with radiation to the ovaries, older age at treatment, treatment with cyclophosphamide or procarbazine, and diagnosis of Hodgkin lymphoma (7). In addition, compared to a sibling cohort, female cancer survivors were less likely to ever become pregnant (relative risk [RR] 0.81; 95% confidence interval [CI]: 0.73 to 0.90) (8). These findings are in-line with older studies including a large National Cancer Institute study of 2283 adult survivors of childhood and adolescent cancer (diagnosed in the period 1945–1975) that included both sexes and a variety of treatment agents (9); the overall crude relative fertility of survivors of cancer as compared with their sibling controls was 0.88. The male survivors had a greater fertility deficit than the female survivors (relative fertility: 0.83 vs. 0.94, respectively). Treatment effects were pronounced, with increased infertility in those treated with a combination of radiation and alkylating agents (3,10). Other studies have also found increased infertility (10). After bone marrow transplant there are high rates of menopause, but some reports show that 29% recover ovarian function (11). Younger age predicted return of ovarian function, whereas total body irradiation had a negative effect (11,12).
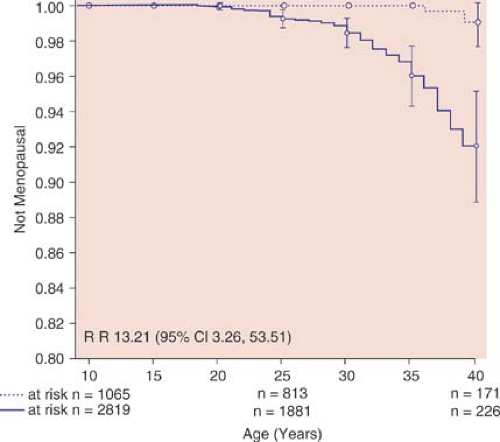
For women with ovarian function after cancer therapy, ovulation may be irregular. Induction of ovulation for attempts of conception should proceed along routine infertility algorithms. There have been reports of induction of ovulation with cancer survivors with abnormal gonadotropin levels with the use of clomiphene citrate and gonadotropins (13,14,15). It is important to counsel long-term cancer survivors that, even if they have regular menses and ovulatory function, they may undergo a premature menopause and they should consider not delaying pregnancies (Fig. 29-1).
Radiation Therapy
Radiation therapy can result in permanent ovarian damage and ovarian failure. In a study of long-term survivors of childhood malignancies, Stillman and colleagues (16) reported that ovarian failure occurred in 68% of patients with both ovaries within the radiation field, in 14% whose ovaries were at the edge of the treatment beam, and in none with one or both ovaries outside the field. Ovarian function is directly related to the dose of radiation and the age of the patient at the time of treatment (17,18,19). The median lethal dose (LD50) for the human oocyte has been estimated to be approximately 400 rad. A single dose of 250 to 500 rad results in menstrual irregularities in all women; up to 60% to 70% of women 15 to 40 years of age and 100% of women older than 40 years of age will be permanently sterilized. Women aged 20 to 30 years can tolerate 2000 rad fractionated over 5 to 6 weeks with less risk of sterility than women older than 40 years of age, in whom 600 rad will induce menopause (20,21,22). Thus, children and adolescents have a better prognosis for preservation of reproductive function than women older than 40 years of age (17,20).
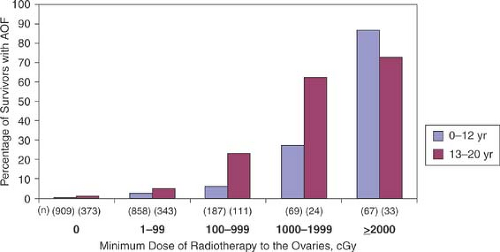
Despite the ability of prepubertal gonads to tolerate higher doses of radiation, total body irradiation (TBI) has a high rate of ovarian failure (55% to 80%) even in this age group. Fractionated dosing has been found to be less toxic than single dosing, even at higher total doses (23). Results from the Childhood Cancer Survivor Study, a cohort of over 20,000 cancer survivors who were treated younger than 21 years of age and survived at least 5 years after diagnosis, indicated that the likelihood of pregnancy was decreased with increasing doses of radiation to the ovaries and uterus. The relative risk of pregnancy in those who received 5 to 10 Gy was 0.56 and those who received >10 Gy was 0.18 compared to a referent group of those not receiving any radiation (8). When analyzing risk factors of acute ovarian failure after treatment, abdominopelvic radiation dose was directly correlated with acute ovarian failure and the risk was directly correlated with age at treatment (Fig. 29-2) (6). In addition, radiation of the prepubertal uterus can lead to a smaller uterine volume in adulthood and increased risk of second-trimester miscarriage if pregnancy does occur spontaneously or with oocyte donation (24). In a long-term follow-up study of girls with acute lymphoblastic leukemia, menarche occurred in the normal age range in 92% of survivors and 96% of controls (25). However, survivors receiving 2400 cGy of craniospinal radiation with or without abdominal radiation had significantly later menarche than controls.
Radiation to areas outside the pelvis can also affect reproductive function. Dysfunction of the hypothalamic–pituitary axis resulting in hypogonadotropic amenorrhea is a common sequela of cranial tumors treated with surgery and/or radiation. Hall and colleagues (26) administered a physiologic replacement regimen of exogenous gonadotropin-releasing hormone (GnRH) to survivors of brain tumors, based on the hypothesis that the defect would be hypothalamic rather than pituitary in origin. Ovulation occurred in 78% of the nine patients. Hyperprolactinemia may also occur secondary to whole-brain irradiation for brain tumors. Early and precocious puberty may occur in girls who received lower doses of hypothalamic–pituitary radiation for acute lymphoblastic leukemia (24,27). Radiation to the
neck can result in hypothyroidism in as many as one-third to one-half of patients who received doses of over 3500 cGy, resulting in delayed maturation and poor linear growth if undiagnosed (28), thus the need for routine screening and follow-up in these patients (see Chapters 8 and 9). The likelihood of pregnancy in childhood cancer survivors receiving >30 Gy of hypothalamic–pituitary radiation is significantly reduced (RR 0.61) compared to no radiation to this region (8).
neck can result in hypothyroidism in as many as one-third to one-half of patients who received doses of over 3500 cGy, resulting in delayed maturation and poor linear growth if undiagnosed (28), thus the need for routine screening and follow-up in these patients (see Chapters 8 and 9). The likelihood of pregnancy in childhood cancer survivors receiving >30 Gy of hypothalamic–pituitary radiation is significantly reduced (RR 0.61) compared to no radiation to this region (8).
Chemotherapy
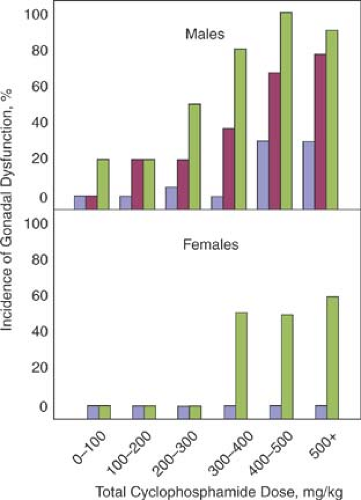
Chemotherapeutic agents affect ovarian function and fertility in a dose-dependent fashion (Fig. 29-3). The marked cytotoxicity of most anticancer drugs, in particular the alkylating agents, can have adverse affects on reproductive potential. Cytotoxic agents produce azoospermia and compromised Leydig cell function in males, and the testicles appear to be more sensitive to these agents than the ovaries (29,30). Cytotoxic agents produce perifollicular agenesis progressing to premature ovarian failure in females (31,32). Chemotherapeutic agents that have been associated with premature ovarian failure in adults include cyclophosphamide, chlorambucil, busulfan, and melphalan (LPAM) and the combination regimens mechlorethamine, vincristine, procarbazine, and prednisone (MOPP); nitrogen mustard, vinblastine, procarbazine, and prednisolone (MVPP); and chlorambucil, vinblastine, prednisolone, procarbazine, doxorubicin, and etoposide (ChlVPP/EVA) (4,16,17,33,34,35).
The severity of gonadal dysfunction is a function of the chemotherapeutic regimen and total dose in both sexes, as well as the age of the patient at the time of therapy (36,37) (Table 29-1). Children and adolescents appear more resistant to the deleterious effects of these agents, although long-term effects have not been fully assessed. In a review of 30 studies that evaluated patients who had received chemotherapy for renal disease (cyclophosphamide), Hodgkin disease, or acute lymphocytic leukemia, Rivkees and Crawford (34) concluded that chemotherapy-induced damage was more likely to occur in sexually mature females and with higher doses of alkylating agents. They found gonadal dysfunction at follow-up in none of the girls given cyclophosphamide during prepuberty and midpuberty compared with 58% of those who were sexually mature. For Hodgkin disease, 7% of those treated in midpuberty and 71% of the sexually mature had gonadal dysfunction. For leukemia, the percentages were 10% for those treated before puberty, 36% for midpuberty, and 22% for those sexually mature. For cyclophosphamide, particularly associated with ovarian damage, a dose of 5.2 g has been associated with amenorrhea in women older than 40 years of age, whereas a dose of 20.4 g has been associated with the same effect in women 20 to 29 years of age (38). Timely menarche does not guarantee preservation of ovarian function. Even in girls who appear to go through a normal or early puberty, prior chemotherapy for acute lymphocytic leukemia can result in elevated follicle-stimulating hormone levels and decreased plasma inhibin levels, despite normal plasma estradiol levels (39,40). Even with resumption of menses after therapy, fertility is impaired in many cancer survivors who received chemotherapy. Premature menopause was associated with increasing alkylating agent dose in survivors of childhood cancer, with the highest-dose alkylating regimens having a relative risk for premature menopause of 5.78 (7).
Combined Chemotherapy and Radiation Therapy
The combination of chemotherapy and radiation therapy, especially in Hodgkin disease, often significantly impairs ovarian function. Patients younger than 30 years of age have the best chance for recovery of ovarian function (41). In a long-term follow-up study of 92 girls treated for Hodgkin disease at age 15 years and older, 87% had normal menstrual function: 83% following pelvic irradiation, 94% following chemotherapy, and 67% following combined modality treatment (42). None of the girls who had subtotal lymphoid irradiation alone (mantle or spade field) or those who received three cycles or less of MOPP developed ovarian failure. Improved fertility rates and better pregnancy outcomes have been noted in patients with Hodgkin disease treated with a limited field of radiation and chemotherapy with thiotepa, vinblastine, vincristine, procarbazine, and prednisone, when compared with the results published in the Horning (41) review of 103 women treated with multiple modalities of chemotherapy with or without total body irradiation after oophoropexy.
Among 1067 five-year cancer survivors with disease diagnosed at 13 to 19 years of age and still menstruating at age 21, Byrne and associates (43) noted the risk of early menopause was four times greater than that of control women 21 to 25 years of age. The relative risk for early menopause was increased with radiation treatment alone (RR 3.7; 95% confidence interval CI: 1.3, 10.0), with alkylators alone (RR 9.2; 95% CI: 2.7, 31.5), and
combined modality of radiation below the diaphragm and alkylating agents (RR 27.4; 95% CI: 12.4, 60.4), when compared to controls. These findings were consistent with those by Sklar and colleagues, who found combined treatment with alkylating agents plus abdominopelvic radiation to be the highest-risk therapy, with a cumulative incidence of nonsurgical premature menopause of nearly 30% in this group (7).
combined modality of radiation below the diaphragm and alkylating agents (RR 27.4; 95% CI: 12.4, 60.4), when compared to controls. These findings were consistent with those by Sklar and colleagues, who found combined treatment with alkylating agents plus abdominopelvic radiation to be the highest-risk therapy, with a cumulative incidence of nonsurgical premature menopause of nearly 30% in this group (7).
Table 29-1 Risks of Permanent Amenorrhea in Women Treated with Modern Chemotherapy and Radiotherapy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Bone Marrow Transplantation
Although bone marrow transplantation (BMT) has been life saving in girls with advanced cancer, as well as for those with metabolic and hematologic diseases, significant gonadal damage may result from preconditioning therapies, which often consist of alkylating agents and/or total body radiation (23,44,45,46,47,48,49). For example, in a large European study of over 37,000 allogenic and autologous stem cell transplants, only 0.6% conceived, half of whom were women, and some used assistive reproductive technology (ART) (50). An analysis of eight studies found the incidence of pubertal or ovarian failure of 22% to 100% (24). Spinelli and colleagues (46) calculated that among 79 females undergoing allogeneic BMT with total body irradiation, the actuarial chance of having a menstrual period at 10 years after BMT was 43%. Four of five girls who received BMT in the premenarcheal age started menses. Immediately after BMT, all adult women had clinical evidence of ovarian insufficiency. Ten (13.5%) of 74 postmenarchal women showed ovarian recovery ranging from 21 to 87 months. Patients younger than 18 years of age had a much better prognosis than those older than 18 years. Girls undergoing bone marrow transplantation for acute lymphoblastic leukemia were found to have normal ovarian function in 50% of cases (51). In a study by Thibaud and colleagues, of 31 girls treated with BMT, only 6 of 31 were reported to have normal ovarian function (23). In a study of 44 postpubertal women after allogeneic BMT, Schubert and co-workers (48) reported that 80% (35 of 44) had reduced vaginal elasticity and rugae; small vaginal, uterine, and cervical size; atrophic vulvovaginitis; introital stenosis; and/or loss of pubic hair. Multiple investigators have found similar results with women having received TBI in childhood having small uterine volumes with poor blood flow, even with hormone replacement therapy (52). Even prepubertal girls treated with BMT for sickle cell disease or thalassemia and given standard preconditioning therapies may have significant gonadal damage (45,49




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree