Diagram 21.1
Fertilization of an empty ovum by sperm containing 23X haploid set of chromosome which duplicates to form 46XX
Paternal Origin of Partial Hydatidiform Mole
Partial hydatidiform moles are triploid and may have a 69XXX, 69XXY, or 69XYY karyotype [3]. PHM results from dispermic fertilization of ovum containing haploid set of 23X chromosome by two sperms containing haploid sets of 23X or 23Y, a diandric triploidy. Fertilization by diploid sperm resulting in partial mole is also suggested (Diagram 21.2).
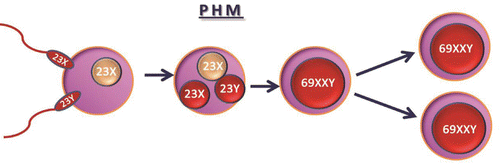

Diagram 21.2
Fertilization of ovum with haploid set of 23X by two sperms with haploid sets of 23X or 23X and 23Y to result in 69XXX or 69XXY. Diandric triploidy
Biparental CHM
The rare type of biparental CHM (BiCHM) is seen in patients with recurrent mole and occurs in families with two or more members of the family developing molar pregnancies. There is contribution from both parents, but due to defect in genomic imprinting, molar pregnancy develops and resembles complete hydatidiform mole. Genetic studies of such families have shown evidence of mutations in the leucine-rich regions of NLRP7 at chromosome 19q13.3-13.4 [8–10]. The mode of inheritance in familial biparental recurrent mole is by an autosomal recessive disorder. The probable mechanism of biparental recurrent moles seems to be a defect in the methylation process in the paternal and maternal alleles in the H19 gene; the paternal allele is methylated and maternal allele is unmythelated. The defective methylation process can sometimes be partial or even may overcome which would explain why in certain woman with the disorder had a normal pregnancy, despite a theoretical recurrence risk of 100 % (Diagram 21.3).
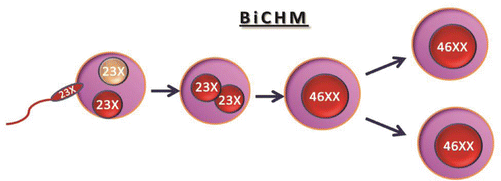

Diagram 21.3
BiCHM is seen in patients with recurrent mole and occurs in families with two or more members developing molar pregnancies. Defect in genomic imprinting, risk of malignancy. Genetic defect – 19q13.3-13.4, NLRP7
Risk Factors
Pregnancy at extremes of age is an important risk factor for development of hydatidiform mole; the risk is doubled in teenage and at age above 35 years. Women older than 40 years experience a 5- to 10-fold increase in risk compared to younger women [11]. Parity does not affect the risk for developing molar pregnancy. Relative deficiency of carotene and animal fat intake is reported as a risk factor. History of previous molar pregnancy is an important risk factor, a reported risk of ten times compared to the normal population. Cigarette smoking, history of infertility, contraceptive use, and induction of ovulation are reported to increase the risk of having molar pregnancy.
Pathology of Trophoblastic Lesions
All types of gestational trophoblastic diseases exhibit proliferation of both syncytiotrophoblast and cytotrophoblast which maintain secretion of hCG. Placental site trophoblastic tumor arising from the intermediate trophoblast cells produces low levels of hCG and hPL.
Complete Hydatidiform Mole
The histological features of complete hydatidiform include:
Generalized diffuse hyperplasia of both cytotrophoblast and syncytiotrophoblast.
Generalized edema of chorionic villi with central cistern formation of varying sizes, which resembles the macroscopic description of the hydatidiform mole, the “bunch of grapes.”
Absenc e of an embryo resorption occurs before development of fetal circulation, and no nucleated fetal erythrocytes are observed in the villous.
Fisher and others in 1997 [12] reported the presence of fetal blood cells in seven complete hydatidiform moles by polymerase chain reaction amplification of DNA. Their conclusion was that fetal blood cells may be present in the villi of complete hydatidiform moles, and the presence of fetal erythrocytes alone should not be considered indicative of a diagnosis of partial hydatidiform mole.
With the availability of ultrasonography for early pregnancy evaluation, many moles are evacuated before the classical development of mole as a case of failed pregnancy. It is important to submit all products evacuated for histopathological study. The classical findings of the complete mole may not be seen in such early cases. Abnormal trophoblastic hyperplasia is a constant finding in early CHM, and the generalized hydrops may not be evident. Early CHM may exhibit stromal blood vessels and stromal karyorrhexis.
Complete moles show overexpression of several growth factors, including c-myc, epidermal growth factor, and c-erb B-2, compared with normal placenta. Immunostaining using p57KIP2 will be absent in complete mole (Fig. 21.1).
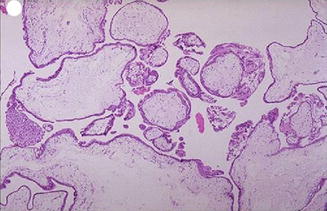

Fig. 21.1
The chorionic villi of complete mole are diffusely hydropic with central cistern formation with no blood vessels and hyperplasia of trophoblastic layers
Partial Hydatidiform Mole
The partial hydatidiform mole (PHM) results by fertilization of an ovum containing haploid set of 23X by two sperms with haploid sets of 23X or 23X and 23Y, a Diandric Triploidy. The characteric features of partial mole are:
Mild focal variable hydropic villi.
Mild trophoblastic hyperplasia, predominantly confined to syncytium.
Fetal vessels are usually present and contain nucleated fetal erythrocytes.
Embryo/fetus will be present often with congenital anomalies.
Runs a milder course and the diagnosis is often missed as “missed abortion”.
It was thought that partial mole does not transform into choriocarcinoma. Recently, Newlands et al. have reported three cases of choriocarcinoma following partial mole [13]. Many other reports of malignant sequelae following PHM were reported by other authors also subsequently [14–16].
The distinction between a hydatidiform mole and an abortion with hydropic villi can be difficult. Like hydatidiform mole, a hydropic abortion may show villous edema with hydropic swelling, although a hydropic abortion lacks the marked trophoblastic hyperplasia. With the introduction of p57kip2 immunohistochemistry, a better differentiation between complete and partial mole has become possible. The p57kip2 gene (CDKN1C) is paternally imprinted and expressed from the maternal allele. Since complete moles lack a maternal genome, p57kip2 immunostaining is absent, whereas hydropic abortus and partial moles show positive staining (Figs. 21.1 and 21.2).
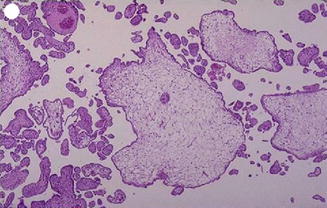

Fig. 21.2
Focal hydropic degeneration with focal hyperplasia of trophoblast with normal villi in between and fetal parts
Invasive Mole
Invasive mole is the penetration of molar villi most often from CHM into the myometrium or the uterine vasculature and may spread to the vagina or lungs. It is locally invasive and can penetrate the full thickness of the myometrium leading to severe intraperitoneal hemorrhage. The lesion is characterized microscopically by trophoblastic invasion of the myometrium with identifiable villous structures. Most invasive moles show moderate to marked trophoblastic activity and persistence of villous structure. The histological diagnosis of invasive mole is rarely made, as hysterectomy is seldom done and it is difficult to identify from curetting. On ultrasound examination it shows focal areas of increased echogenicity within the myometrium. The lesion is heterogeneous containing small fluid-filled cavities, and Doppler color flow mapping shows high vascularity. It is associated with uterine subinvolution, persistent heavy vaginal bleeding, and rising hCG level. Chemotherapy may be started on the basis of rising hCG level, and it is not necessary to confirm the histopathological diagnosis of invasive mole or choriocarcinoma (Figs. 21.3, 21.4, 21.5, 21.6, and 21.7).
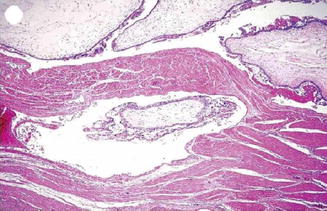
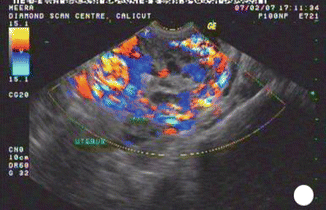
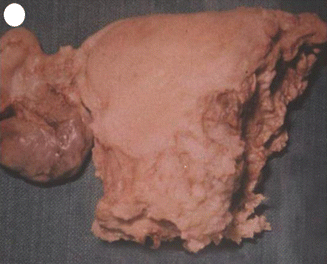
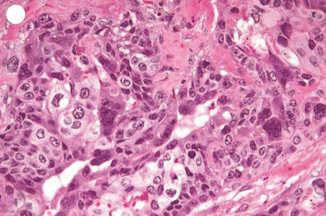
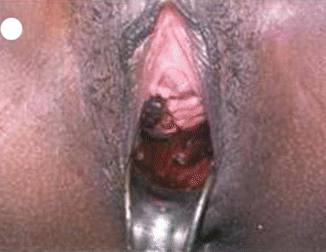

Fig. 21.3
Invasive mole – trophoblastic element is invading into the myometrium maintaining the villous pattern

Fig. 21.4
Color Doppler study showing highly vascularized area in the myometrium in invasive mole

Fig. 21.5
Subtotal hysterectomy done as a life-saving procedure for perforation of uterus by invasive mole which leads to severe intraperitoneal bleeding

Fig. 21.6
Choriocarcinoma showing both cytotrophoblastic and syncytiotrophoblastic hyperplasia with atypia

Fig. 21.7
Suburethral nodule causing vaginal bleeding in GTN
Ectopic Hydatidiform Mole
Rarely molar pregnancy may develop in the fallopian tube. The criteria for diagnosis are the same as for hydatidiform mole present in the uterine cavity. Sonographically it will be seen as an ectopic pregnancy.
Gestational Choriocarcinoma
Choriocarcinoma is a malignant neoplasm consisting of sheets of anaplastic cytotrophoblasts and syncytiotrophoblasts without chorionic villi. Some intermediate trophoblasts may also be seen. The important risk factor for choriocarcinoma is hydatidiform mole, especially following complete mole. The risk of developing choriocarcinoma after partial mole is rare. Choriocarcinoma can develop after normal pregnancy, abortion, and ectopic pregnancy. In some cases there is no known antecedent pregnancy, and it is postulated that choriocarcinoma may develop from a conceptus ab initio [17]. It stimulates virtually no stromal reaction and is therefore essentially a mixture of hemorrhage and necrosis with tumor cells scattered within the mass. On microscopic examination, viable tumor is usually confined to the rim of the neoplasm as choriocarcinoma lacks an intrinsic vasculature; the tumor cells derive nutrition by invasion of maternal blood vessels. Widespread intravascular dissemination to lungs, brain, and other sites is common. The metastatic sites have a tendency to rapidly outgrow their blood supply resulting in necrosis and hemorrhage. Extensive necrosis, hemorrhage, and vascular invasion are common. The commonest sites for metastasis are lung, brain, liver, pelvis, vagina, spleen, intestine, and kidney (Figs. 21.6, 21.8, and 21.9).
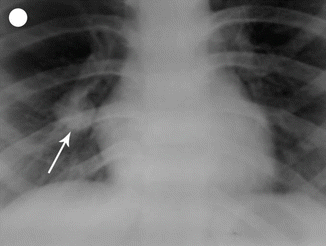
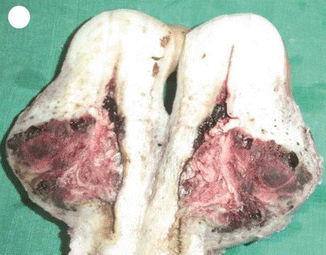

Fig. 21.8
Chest X-ray showing secondary in the lung

Fig. 21.9
Choriocarcinoma of the uterus
Placental Site Trophoblastic Tumor (PSTT)
In 1981, Scully and others proposed placental site trophoblastic tumor (PSTT) to describe a variant of gestational trophoblastic tumor [18]. It is a rare type of GTN and is composed mainly of intermediate cytotrophoblastic cells arising from the placental implantation site. These tumors can result from any type of antecedent pregnancy and are locally invasive and less widely metastatic. Placental site trophoblastic tumor produces more of hPL than hCG as it contains less syncytiotrophoblast, and hence hCG may not serve as a reliable tumor marker for follow-up. However clinically the diagnosis should be suspected when hCG levels are low relative to the tumor burden. It is a slow-growing tumor with late metastasis and involvement of lymph nodes and produces low levels of free β-hCG.
PSTT is generally seen as a proliferation of extravillous or intermediate trophoblast in the myometrium or endomyometrium. Chorionic villi are rarely found and the typical dimorphic pattern of choriocarcinoma is absent. Instead, there is a characteristic pattern consisting of a relatively monomorphic population of predominantly mononuclear trophoblastic cells infiltrating the myometrium and splitting apart the muscle fibers. Necrosis is more prominent in PSTT as opposed to hemorrhage in choriocarcinoma and vascular invasion is not as common as in choriocarcinoma. Immunohistochemical staining for human placental lactogen (hPL), CD146 (MEL-CAM), and placental alkaline phosphatase (PLAP) are additional diagnostic tests for PSTT that have a specificity of approximately 60 % [19]. PSTT usually presents with amenorrhea or irregular vaginal bleeding months or years after a normal pregnancy, an abortion, or, rarely, a hydatidiform mole [20]. Origin from both moles and normal pregnancy has been demonstrated genetically [21, 22]. Response to chemotherapy is poor in PSTT and stage one disease is better treated with hysterectomy. Metastatic disease is started on with multi-agent chemotherapy.
Epithelioid Trophoblastic Tumor (ETT)
Epithelioid trophoblastic tumor is a rare neoplasm and is the most recent addition to the gestational trophoblastic tumor category [23]. Most of the patients reported in the literature were women of reproductive age, and the antecedent pregnancy event was full-term deliveries, spontaneous abortions, and molar gestations. The average interval between the antecedent gestation and the development of the tumor is 5–6 years.
Only 52 cases were reported till 2008 [24]. It is composed of chorionic-type intermediate trophoblast and is distinct from choriocarcinoma and PSTT. The tumor is composed of sheets and nests of mononuclear trophoblast with clear, eosinophilic, and vacuolated cytoplasm resembling “chorionic-type” intermediate trophoblast. Histologically, epithelioid trophoblastic tumor is a distinct neoplasm whose cytological features and growth patterns mimic squamous cell carcinoma. Gross inspection of ETT shows a solid to cystic, fleshy, and well-defined mass in the uterine wall, lower uterine segment, or endocervix. It can be confused with squamous cell carcinoma because of its frequent involvement of the lower uterine segment or endocervix and its epithelioid histologic appearance and expression of p63 and cytokeratins (Figs. 21.10 and 21.11).
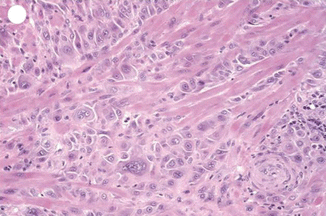
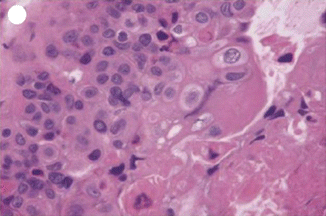

Fig. 21.10
PSTT showing proliferation with atypia of extravillous trophoblast splitting apart muscle fibers

Fig. 21.11
ETT – showing epithelioid intermediate trophoblastic tumor cells and eosinophilic hyalinization (keratin-like material) within tumor nests, simulating invasive squamous cell carcinoma
Clinical Presentation of Molar Pregnancy
Patients with molar pregnancy may present with excessive nausea and vomiting in early weeks. Vomiting may be severe enough requiring hospitalization for correction of fluid and electrolyte imbalance. Most patients with hydatidiform mole will have vaginal bleeding, which may be sudden and profuse. Previously reported features such as anemia, uterine enlargement more than for the period of amenorrhea, preeclampsia, hyperemesis, hyperthyroidism, and respiratory distress are rarely seen nowadays due to early termination of failing pregnancy due to routine use of ultrasonography. The symptoms in partial mole are very mild and late to start, and may be diagnosed as missed abortion. The routine use of ultrasonography in first trimester will diagnose cases of hydatidiform mole as blighted ovum or failed pregnancy and may be terminated without a diagnosis. Even today, patients without proper antenatal care may report at a more advanced gestational age with excessive uterine size, large theca lutein cysts, and symptoms of hyperthyroidism, early onset preeclampsia, and anemia.
Ultrasound in Diagnosis of H. Mole
Characteristic ultrasonographic scans of complete mole show a uterine cavity filled with a heterogeneous mass with anechoic spaces of varying size and shape, a snowstorm-like appearance without any fetal parts. Theca lutein ovarian cysts secondary to high hCG level may be present in about 30 % of cases, where the pregnancy has reached 14–18 weeks. With early detection and termination of failing pregnancy, these classic sonographic findings will be absent. However, the majority of first trimester complete moles still demonstrate a typical ultrasound appearance of a complex, echogenic intrauterine mass containing multiple small cystic spaces [25]. In about 30 % of partial moles, the sonographic diagnosis will be possible; the rest may be diagnosed as missed abortion. The ultrasound findings observed in partial mole are an excessively enlarged placenta, cystic spaces within the placenta, gestational sac which is either empty or containing amorphous echoes, or a growth retarded fetus.
Ancillary techniques are needed in some cases to differentiate non-molar miscarriage from hydatidiform mole, including immunostaining for P57kip2, the product of CDKN1C. P57kip2 is expressed by the maternal allele and is visible on histology as nuclear staining of cytotrophoblast and villous mesenchyme in placenta of all gestations apart from androgenetic complete mole [26] (Figs. 21.12, 21.13, and 21.14).
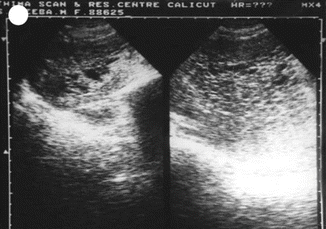
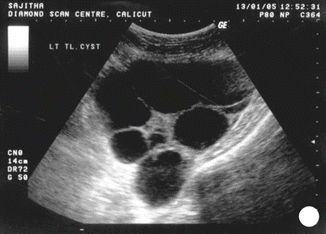
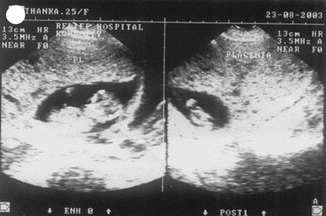

Fig. 21.12
USG showing complete mole

Fig. 21.13
USG showing theca lutein cyst in complete mole

Fig. 21.14
USG showing partial mole
In cases of a coexisting normal fetus in a twin pregnancy with mole and in cases of placental mesenchymal dysplasia, a mistaken diagnosis of partial mole is possible and in both cases the careful repeated examination and use of 3-D ultrasound will help to make a correct diagnosis. Amniocentesis and fetal karyotyping will confirm the diagnosis as in partial mole, the baby will be triploid (Figs. 21.6, 21.15, 21.16, and 21.17).
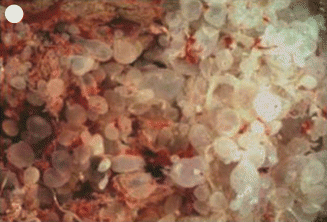
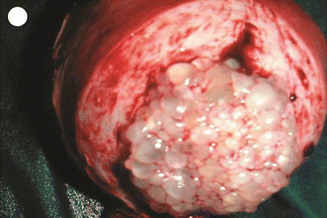
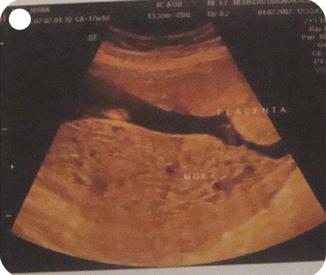

Fig. 21.15
Moles evacuated by suction

Fig. 21.16
Hysterectomy with mole in situ

Fig. 21.17
3-D ultrasound showing molar tissue and normal placenta in a case of twin with one mole
All products of conception from nonviable pregnancies should undergo histological examination irrespective of ultrasonographic findings, and all patients after medical termination of pregnancy should have urine hCG tested after 4–6 weeks to rule out any persistent trophoblastic activity.
Management of Hydatidiform Mole
When a diagnosis of complete or partial molar pregnancy is made, the most appropriate method of evacuation should be decided. Assessment of the patient for the presence of medical complications, including anemia, preeclampsia, hyperthyroidism, and respiratory insufficiency, is essential. It is appropriate to have a baseline pre-evacuation hCG, and a chest X-ray may be taken in addition to routine blood and urinalysis. The primary management of hydatidiform mole is evacuation of the mole. Crossmatched blood for transfusion and appropriate fluid resuscitation measures should be kept ready at the time of evacuation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


