The varus and valgus moments generated at the knee can be even more substantial during sporting activities such as running, side-stepping, and cutting6 and can serve as a predictor of injury risk. Elevated valgus moments during landing from a jump have been identified as a predictor of anterior cruciate ligament (ACL) injury risk in female athletes.7
Frontal Plane Stability and the Role of Muscles
The bicondylar shape of the distal femur is well-suited to resisting the varus and valgus moments that act on the knee during daily activities and sports. When the knee compresses due to muscle contraction and/or the ground reaction force, an applied moment in the frontal plane will cause redistribution of the contact force acting on the medial and lateral condyles.8,9 The ability of the articular surfaces to resist the applied moment is proportional to the magnitude of the contact force and its distance from the joint center. This highlights the importance of the muscles in generating contact forces that increase the ability of the articular surfaces to resist frontal plane moments.
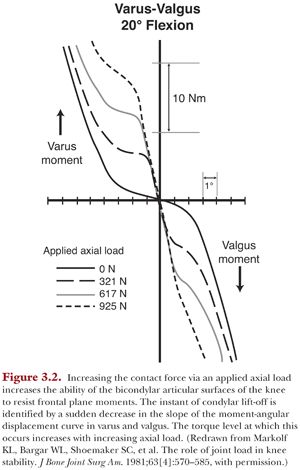
The articular surfaces can rapidly generate resistive frontal plane moments with minimal varus or valgus angulation (≤2°) in the presence of a compressive load (Fig. 3.2). The small amounts of angulation are due to compression of the cartilage and menisci. Condylar lift-off will occur when the applied moment has exceeded the capacity of the articulating surfaces to generate an internal counteracting moment. This instant is characterized by a sudden flattening in the slope of the moment-angular displacement response (see Fig. 3.2). As the knee continues to rotate into varus or valgus, the ligaments will engage and generate forces that cause the resistive moment to once again increase (see Fig. 3.2).
Stability Provided by the Articulating Surfaces
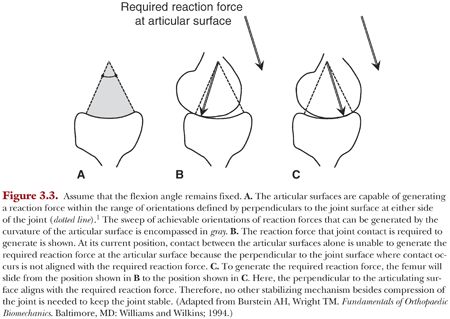
The shapes of the articulating surfaces of the knee provide an involuntary mechanism to provide joint stability.1 The joint will unconsciously move to a contact position that can counter the direction of an applied force within an envelope defined by the size and shape of the articular surfaces (Fig. 3.3). The bounds of this envelope can be visualized by drawing lines that are perpendicular to the articulating surfaces at the extreme ends of the joint (Fig. 3.3A).1 The directions of these bounding lines encompass all orientations of reaction forces that can be generated by the articulating surfaces. Thus, the articular surfaces may accommodate changes in the direction of the applied loads through compression of the joint alone and by adjusting the relative position of the joint surfaces (Fig. 3.3 B,C).
Because the knee is conforming in the coronal plane, it takes only small shifts in the mediolateral position of the knee to produce mediolateral-directed contact forces (Fig. 3.4).
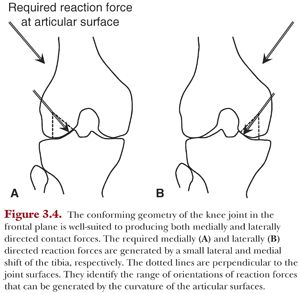
Similarly, the medial compartment of the knee will resist small changes in anterior-posterior–oriented forces in the sagittal plane due to its concave shape. The lateral compartment, however, based on its convex shape in the sagittal plane and lateral orientation in the frontal plane, cannot effectively resist anteriorly and medially directed external forces, respectively. Thus, it requires additional stabilizing mechanisms to accommodate these applied forces including the menisci and the ACL.
Stability Provided by Ligaments
The forces generated by ligaments also contribute to joint stability. However, when acting to restrain the joint, they must not interfere with the normal range of motion of the knee. This is accomplished because their line of action passes close to the rotation axis of the joint.1 This makes it impossible to generate large resistive moments against flexion and extension because the distance between rotation axis and the line of action of the force generated by the ligament remains small through flexion. When this principle is not adhered to during ligament reconstruction, for example, it can lead to clinical complications including flexion contracture and overconstraint of the joint.
Ligaments must also allow some relative movement of the articular surfaces for the articular stabilizing mechanism discussed earlier to be effective.1 Two characteristic features of ligaments facilitate this: their slack length and toe region. Slack length describes the length at which the ligament begins to carry load. The toe region refers to the initial portion of the load-displacement response of the ligament where load builds slowly as it is stretched. These two features allow the ligament to gradually generate force for the first few millimeters of relative movement of the joint surfaces. This enables the articular surfaces to move to a suitable contact point along their curvature to generate the required reaction force (Fig. 3.5).
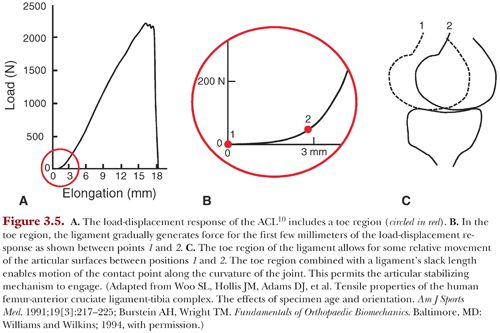
If there is no suitable point along the curvature of the articular surfaces that can generate the required reaction force, the bones displace further apart. As the bones displace further, the ligaments will generate force more rapidly as they stiffen.10 Thus, they will make a larger contribution to resisting loads applied to the joint.
Finally, a ligament should be oriented as closely as possible in the direction of the applied force that it must resist.1 Thus, the collateral ligaments are appropriately oriented to resist frontal plane moments based on their mediolateral distance from the joint center and proximal-distal orientation. Similarly, the ACL has an oblique orientation with origin on the medial aspect of the lateral femoral condyle and insertion on the anterior aspect of the tibia. Thus, its orientation reveals its function in resisting forces that act to push the tibia anteriorly and medially.11–13 Its small offset relative to the center of the joint also makes it a secondary stabilizer against varus and valgus.13
Knee Instability
Situations arise where the stabilizing mechanisms described earlier fail; that is, they cannot effectively counteract the magnitude and direction of the externally applied loads. This will lead to instances where the knee joint feels unstable. These instability events, or episodes where the knee gives way, compromise function and can lead to cartilage and meniscal damage.14,15 Relief from knee instability is a primary reason why patients seek treatment for ACL injury.
Instability has been defined as large changes in the position of articular contact with small changes in applied load.1 A stable joint is one where the articular surfaces glide smoothly relative to each other without sudden jumps or abrupt changes in their relative position. Instability can occur when a stabilizing mechanism is absent (e.g., ACL insufficiency) or when existing stabilizing mechanisms are incapable of providing an appropriate resistive force. This might happen when a movement occurs too quickly for the muscles to generate the required force such as in a quick cutting maneuver while playing sports. Stabilizing mechanisms might also fail in combination such as muscle weakness combined with a ligament injury leading to a giving-way episode in an ACL-deficient patient.
Episodes of giving way in ACL-deficient individuals have been serendipitously recorded in the gait laboratory.16,17 This provides a unique opportunity to quantify the time sequence of loads and motions experienced by the knee over the course of this event. A moderate giving-way episode suffered by an individual while descending stairs revealed that an abnormally high applied valgus moment on the tibia preceded the instability event.16 The instability event was associated with large and rapid anterior translation of the tibia relative to the femur and increased knee flexion compared to successfully performed trials. Another giving-way episode recorded during a cutting activity was preceded by reductions in the applied varus moment.17 This was followed by a transition from an external rotation moment to an internal rotation moment acting on the tibia. These abnormal moments were followed by increased internal rotation of the tibia relative to the femur and increased flexion of the knee compared to successful cutting trials.
These unique data bear the hallmark signs of the clinical pivot shift, which is also generated by a complex combination of applied valgus and internal rotation moments combined with knee flexion.14,15 This reveals the major function of the ACL as a restraint to anterolateral subluxation of the tibia against moments that tend to abduct and internally rotate the tibia.18,19
Interestingly, these giving-way episodes were rapid. Therefore, the restraint provided by muscle forces was likely low because the instability occurred too quickly to be resisted by a voluntary muscle response.20 This indicates that the remaining articular and ligamentous passive stabilizers in the ACL-deficient knee were unable to maintain the position of the knee under these multiplanar loading conditions.
A closer inspection of the interaction of the articular and ligamentous stabilizers against the applied valgus and internal rotation moments provides insight into a potential cause of the instability event. This example ties together our discussion of stabilizing mechanisms of the knee focusing on the role of the ACL. First, the valgus moment acting on the tibia gives rise to a force on the convex lateral compartment that has both anterior and compressive components. Then, an internal rotation moment acts to drive the lateral compartment of the tibia anteriorly. The ACL is oriented to resist these anterior forces. However, in the ACL-deficient knee, this stabilizing mechanism is not available, and the lateral compartment of the tibia will be subject to an abrupt anterior translation relative to the femur. Only when secondary stabilizers such as the lateral meniscus engage to provide the needed posterior reaction force on the tibia will the lateral compartment cease translating anteriorly.
ANATOMY AND BIOMECHANICAL FUNCTION OF THE ANTERIOR CRUCIATE LIGAMENT
The ACL has classically been divided into two functional bundles: an anteromedial and posterolateral portion. This terminology stems from the relative location of its tibial insertion and was described initially by Abbot and colleagues21 and later by Girgis et al.22 This description has proven convenient in grossly demarcating function of major portions of the ACL; however, these bundles may not be easily identifiable in all individuals.23,24 Assessment of the material properties of these bundles indicated that the elastic modulus and the maximum stress of the anteromedial bundle is about 45% greater and at least twice as high as that of the posterolateral bundle, respectively.25 Taken to its logical extreme, the ACL could be further subdivided.26,27 In fact, the ACL acts as a continuum of fibers28 and has been divided into as many as 20 smaller bundles each about 1 mm in diameter.27
The ACL appears flat in extension and fans out about 10 to 12 mm distal to the femoral attachment,22 thus forming a flat hourglass shape along its length.29 This specialized shape enables the ACL to achieve an anterior and medial orientation to allow it to effectively resist loads in these directions while also accommodating the femoral notch.
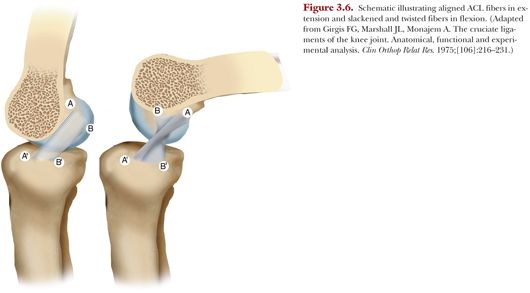
Early observations by Groves30 indicated that the bulk of the ACL was taut in extension, and this was later corroborated by Girgis et al.22 The ACL twists on itself as the orientation of the femoral attachment changes from more vertical in extension to more horizontal in flexion.22 The tissue inserting most proximal and anterior into the femur remains tight on visual inspection while the tissue inserting more distally into the femur slackens22 (Fig. 3.6).
Pioneering work by Artmann and Wirth31 quantified the relative changes in distance between the tibial insertion and various points on the femoral insertion through flexion by monitoring the change in length of cables spanning the tibial and femoral insertions. They reported that anteroproximal locations on the femur were isometric. This approach has been reproduced by others with similar results.32 However, such studies rely on measurement of the straight-line distance between tibia and femur when in fact the individual ACL fibers wrap around one another and do not follow a linear path.27 This was recognized and elegantly addressed by Amis and Dawkins23 by traversing wires through the tibial insertion along fiber bundles comprising the anteromedial bundle, posterolateral bundle, and an intermediate bundle between them. The wires were fixed in the femoral insertion of each bundle. The length changes of these wires were then measured while the knee was passively flexed from full extension to 130 degrees flexion. None of the three fiber bundles was isometric through flexion23 (Fig. 3.7). However, the anteromedial bundle did experience smaller changes in length (~3.5 mm) than the posterolateral bundle (~5 mm) from full extension to 130 degrees flexion.
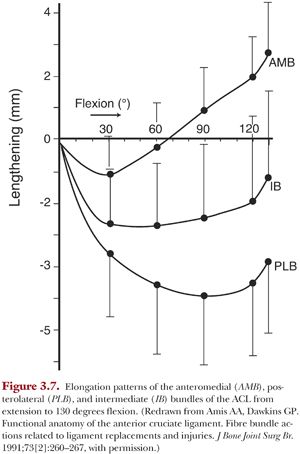
Importantly, the posterolateral bundle was shown to undergo rapid shortening between full extension and 30 degrees flexion. This shortening was recognized as buckling collapse of the ligament.33 Finally, length changes also varied up to 2 mm from specimen to specimen.23
These findings have implications for reconstruction of the ACL. Grafts placed at the insertion of the posterolateral bundle will be less isometric through flexion. Therefore, if fixed in flexion, they will elongate as the knee is extended. This may predispose them to high strains and elevated loads.
The high variability in length changes from specimen to specimen indicates that elongation patterns in some patients will be more sensitive to graft fixation angle. Finally, grafts cover an area of the femoral insertion, not a single point, so different regions of the graft will experience different elongation patterns based on tunnel location even if a portion of the graft encompasses an isometric point. Thus, a graft will experience varying strain patterns through its cross-section.
The anteromedial and posterolateral portions of the ACL have differential roles in resisting externally applied anterior and rotatory loads.34 For both loading cases, the anteromedial bundle bears load throughout flexion, whereas the posterolateral bundle bears the most load near extension. In response to an anterior applied force, the loads borne by the anteromedial portion of the ACL increased from full extension to 60 degrees flexion and then decreased. In contrast, the loads generated in the posterolateral portion of the ACL under an anterior force were highest at full extension and 15 degrees flexion but then decreased from 30 to 120 degrees flexion. At 90 degrees and greater, load in the posterolateral bundle was minimal (<10 N) in response to an anterior load. Under combined valgus and internal rotation moments, the load borne by the anterolateral and posteromedial bundles was about equal at 15 degrees flexion, whereas at 30 degrees flexion, the anteromedial bundle bore two and a half times more load than the posterolateral bundle.
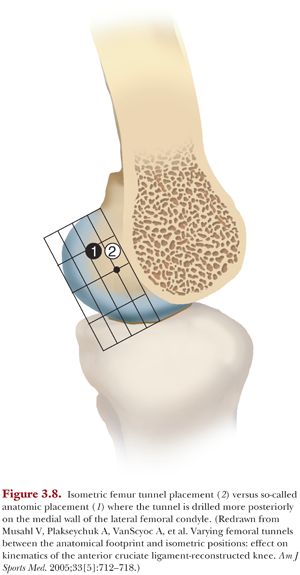
Much research since the early 2000s has been devoted to evaluating ACL reconstructions that strive to recreate the differential function of the anteromedial and posterolateral bundles. Given that malposition of the femoral tunnel is a common cause of ACL graft failure, femoral tunnel placement continues to be an important consideration in ACL reconstruction.35 This approach has evolved into an ACL reconstruction using either one or two grafts placed within the footprint of the native ACL, a so-called anatomic reconstruction.36 In vitro biomechanical studies indicated the ability of double-bundle ACL reconstruction to better control coupled anterior translations in response to combined valgus and internal rotation moments (a so-called simulated pivot shift) than isolated reconstruction of the anteromedial bundle.37 Furthermore, a single quadrupled hamstring tendon graft placed more posteriorly within the femoral footprint restored coupled anterior tibial translations closer to the ACL intact knee than a graft placed in a location that was more isometric38 (Fig. 3.8).