 Figure 23–1. Body water compartments related to age. (Modified, with permission, from Friis-Hansen B: Body water compartments in children: changes during growth and related changes in body composition. Pediatrics 1961;28:169.)
Figure 23–1. Body water compartments related to age. (Modified, with permission, from Friis-Hansen B: Body water compartments in children: changes during growth and related changes in body composition. Pediatrics 1961;28:169.)
The principal constituents of plasma are sodium, chloride, bicarbonate, and protein (primarily albumin). The ISF is similar to plasma but lacks significant amounts of protein (Figure 23–2). Conversely, the ICF is rich in potassium, magnesium, phosphates, sulfates, and protein.
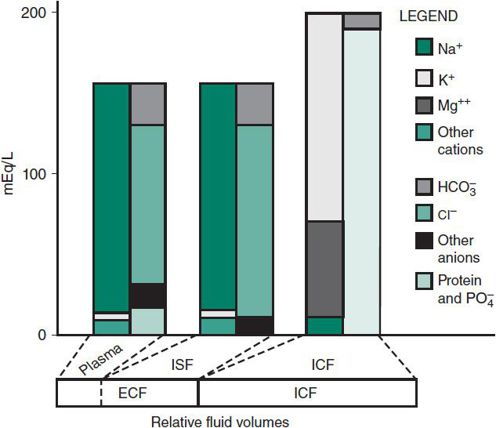
 Figure 23–2. Composition of body fluids. ECF, extracellular fluid; ICF, intracellular fluid; ISF, interstitial fluid.
Figure 23–2. Composition of body fluids. ECF, extracellular fluid; ICF, intracellular fluid; ISF, interstitial fluid.
An understanding of osmotic shifts between the ECF and ICF is fundamental to understanding disorders of fluid balance. Iso-osmolality is generally maintained between fluid compartments. Because the cell membrane is water-permeable, abnormal fluid shifts occur if the concentration of solutes that cannot permeate the cell membrane in the ECF does not equal the concentration of such solutes in the ICF. Thus, NaCl, mannitol, and glucose (in the setting of hyperglycemia) remain restricted to the ECF space and contribute effective osmoles by obligating water to remain in the ECF compartment. In contrast, a freely permeable solute such as urea does not contribute effective osmoles because it is not restricted to the ECF and readily crosses cell membranes. Tonicity, or effective osmolality, differs from measured osmolality in that it accounts only for osmotically active impermeable solutes rather than all osmotically active solutes, including those that are permeable to cell membranes. Osmolality may be estimated by the following formula:
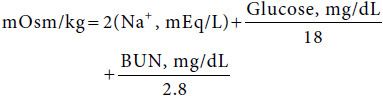
Although osmolality and osmolarity differ, the former being an expression of osmotic activity per weight (kg) and the latter per volume (L) of solution, for clinical purposes they are similar and occasionally used interchangeably. Oncotic pressure, or colloid osmotic pressure, represents the osmotic activity of macromolecular constituents such as albumin in the plasma and body fluids. The importance of albumin in maintaining intravascular volume status is reflected in the setting of the nephrotic syndrome, protein losing enteropathy, and other low serum albumin states wherein fluids accumulate in the interstitial compartment leading to pitting edema.
The principal mechanisms that regulate ECF volume and tonicity are thirst, vasopressin or antidiuretic hormone (ADH), aldosterone, and atrial natriuretic factor (ANF), the latter three exerting their influence by their effects on renal water and sodium handling.
Thirst
Water intake is commonly determined by cultural factors rather than by thirst. Thirst is not physiologically stimulated until plasma osmolality reaches 290 mOsm/kg, a level at which ADH levels are sufficient to induce maximal antidiuresis. Thirst provides control over a wide range of fluid volumes and can even be a response to an absence of or lack of responsiveness to ADH, which results in the production of copious, dilute urine, as in central or nephrogenic diabetes insipidus, or other ADH unresponsive states such as obstructive uropathy. One who cannot perceive thirst develops profound problems with fluid balance.
Antidiuretic Hormone
In the kidney, ADH increases water reabsorption in the cortical and medullary collecting ducts, leading to formation of concentrated urine. In the absence of ADH, dilute urine is produced. Under normal conditions, ADH secretion is regulated by the tonicity of body fluids rather than the fluid volume and becomes detectable at a plasma osmolality of 280 mOsm/kg or greater. However, tonicity may be sacrificed to preserve ECF volume, as in the case of hyponatremic dehydration, wherein ADH secretion and renal water retention are maximal.
Aldosterone
Aldosterone is released from the adrenal cortex in response to decreased effective circulating volume and stimulation of the renin-angiotensin-aldosterone axis or in response to increasing plasma K+. Aldosterone enhances renal tubular reabsorption of Na+ in exchange for K+, and to a lesser degree H+. At a constant osmolality, retention of Na+ leads to expansion of ECF volume and suppression of aldosterone release.
Atrial Natriuretic Factor
ANF, a polypeptide hormone secreted principally by the cardiac atria in response to atrial dilation, plays an important role in regulation of blood volume and blood pressure. ANF inhibits renin secretion and aldosterone synthesis and causes an increase in glomerular filtration rate and renal sodium excretion. ANF also guards against excessive plasma volume expansion in the face of increased ECF volume by shifting fluid from the vascular to the interstitial compartment. ANF inhibits angiotensin II– and norepinephrine-induced vasoconstriction and acts in the brain to decrease the desire for salt and inhibit the release of ADH. Thus, the net effect of ANF is a decrease in blood volume and blood pressure associated with natriuresis and diuresis.
Finberg L et al: Water and Electrolytes in Pediatrics: Physiology, Pathophysiology and Treatment, 2nd ed. WB Saunders; 1993.
Friedman A: Fluid and electrolyte therapy: a primer. Pediatr Nephrol 2009;7:1189 [PMID: 19444484].
ACID-BASE BALANCE
The pH of arterial blood is maintained between 7.38 and 7.42 to ensure that pH-sensitive enzyme systems function normally. Acid-base balance is maintained by interaction of the lungs, kidneys, and systemic buffering systems. Over 50% of the blood’s buffering capacity is provided by the carbonic acid–bicarbonate system, roughly 30% by hemoglobin, and the remainder by phosphates and ammonium. The carbonic acid–bicarbonate system, depicted chemically as
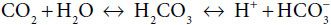
interacts via the lungs and kidneys, and in conjunction with the nonbicarbonate systems, to stabilize systemic pH. The concentration of dissolved CO2 in blood is established by the respiratory system and that of HCO3– by the kidneys. Disturbances in acid-base balance are initially stabilized by chemical buffering, compensated for by pulmonary or renal regulation of CO2 or, respectively, and ultimately corrected when the primary cause of the acid-base disturbance is eliminated.
Renal regulation of acid-base balance is accomplished by the reabsorption of filtered, primarily in the proximal tubule, and the excretion of H+ or HCO3– in the distal nephron to match the net input of acid or base. When urine is alkalinized, HCO3– enters the kidney and is ultimately lost in the urine. Alkalinization of the urine may occur when an absolute or relative excess of bicarbonate exists. However, urinary alkalinization will not occur if there is a deficiency of Na+ or K+, because HCO3– must also be retained to maintain electroneutrality. In contrast, the urine may be acidified if an absolute or relative decrease occurs in systemic HCO3–. In this setting, proximal tubular HCO3– reabsorption and distal tubular H+ excretion are maximal. A “paradoxical aciduria” with low urinary pH may be seen in the setting of hypokalemic metabolic alkalosis and systemic K+ depletion wherein H+ is exchanged and excreted in preference to K+ in response to mineralocorticoid. Some of the processes involved in acid-base regulation are shown in Figure 23–3.
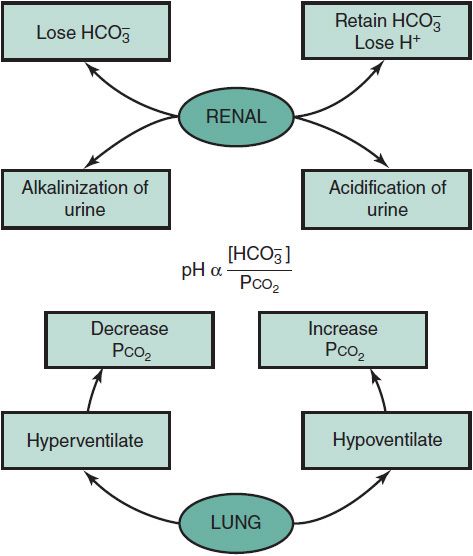
 Figure 23–3. Maintaining metabolic stability via compensatory mechanisms.
Figure 23–3. Maintaining metabolic stability via compensatory mechanisms.
Fall PJ: A stepwise approach to acid-base disorders. Practical patient evaluation for metabolic acidosis and other conditions. Postgraduate Med 2000;107:249 [PMID: 10728149].
FLUID & ELECTROLYTE MANAGEMENT
Therapy of fluid and electrolyte disorders is directed toward providing maintenance fluid and electrolyte requirements, replenishing prior losses, and replacing persistent abnormal losses. Therapy should be phased to (1) rapidly expand the ECF volume and restore tissue perfusion, (2) replenish fluid and electrolyte deficits while correcting attendant acid-base abnormalities, (3) meet the patient’s nutritional needs, and (4) replace ongoing losses.
The cornerstone of therapy involves an understanding of maintenance fluid and electrolyte requirements. Maintenance requirements call for provision of enough water, glucose, and electrolytes to prevent deterioration of body stores for a euvolemic patient. During short-term parenteral therapy, sufficient glucose is provided to prevent ketosis and limit protein catabolism, although this usually provides little more than 20% of the patient’s true caloric needs. Prior to the administration of maintenance fluids, it is important to consider the patient’s volume status and to determine whether intravenous fluids are truly needed.
Various models have been devised to facilitate calculation of maintenance requirements based on body surface area, weight, and caloric expenditure. A system based on caloric expenditure is most helpful, because 1 mL of water is needed for each kilocalorie expended. The system presented in Table 23–1 is based on caloric needs and is applicable to children weighing more than 3 kg.
Table 23–1. Caloric and water needs per unit of body weight.
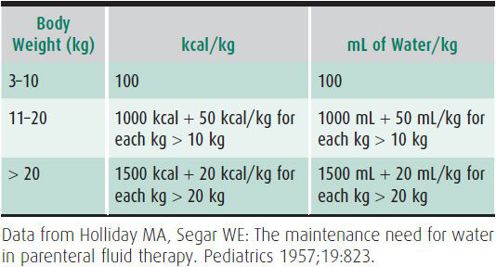
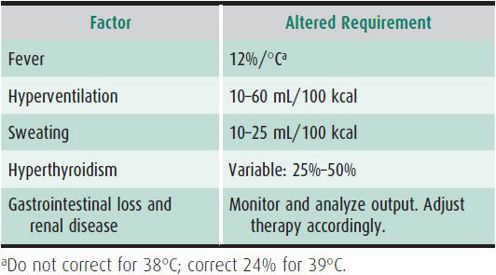
As depicted in Table 23–1, a child weighing 30 kg would need 1700 kcal or 1700 mL of water daily. If the child received parenteral fluids for 2 days, the fluid would usually contain 5% glucose, which would provide 340 kcal/d, or 20% of the maintenance caloric needs. Maintenance fluid requirements take into account normal insensible water losses and water lost in sweat, urine, and stool, and assume the patient to be afebrile, at their true dry weight, and relatively inactive. Thus, if excessive losses occur, standard “maintenance fluids” will be inadequate. In contrast, if losses are reduced for any reason, standard “maintenance fluid” administration would be excessive. Maintenance requirements are greater for low-birth-weight and preterm infants. Table 23–2 lists other factors that commonly alter fluid and caloric needs.
Table 23–2. Alterations of maintenance fluid requirements.
Electrolyte losses occur primarily through the urinary tract and to a lesser degree via the skin and stool. Although maintenance sodium and potassium electrolyte needs have historically been approximated to be in the 3 mEq Na/100 kcal and 2 mEq K/100 kcal range, respectively, leading to the common use of intravenous fluids with 30–40 mEq/L of sodium (1/4 normal saline) and 20 mEq/L of potassium, over the past 10 years Moritz and Ayus have drawn attention to the very serious problem of hospital-acquired hyponatremia in children with the use of hypotonic IV solutions. It is notable, that hyponatremia is the most common electrolyte abnormality in children and affects ~ 25% of hospitalized pediatric patients. It is also important to emphasize that the astute clinician will bear in mind the dynamic nature of clinical context in treating patients. A child with profound water loss stools and hypernatremia who is placed on hypotonic IV fluids and whose diarrhea ceases, but is continued on hypotonic solution without close monitoring of serum electrolytes is at grave risk for the devastating clinical consequences that may develop in the setting of hyponatremia, such as seizures and neurologic impairment. Although stress-induced nonosmotic release of vasopressin and associated increased free water retention is certainly a factor in the development of hospital-acquired hyponatremia in children, 3 mEq Na/100 kcal underestimates daily sodium needs by about half. True needs more closely approximate 5–6 mEq/100 kcal in these clinical situations. In recent years, there also has been a trend for total parenteral nutrition solution sodium, and other electrolytes to be calculated and ordered on a milliequivalents-per-kilogram basis rather than the more classic milliequivalents-per-liter basis (eg, 0.2 or 0.45 normal). It is extremely important to understand that if the administered fluid volume is decreased in this setting, as the child is weaned from supplemental IV fluids to enteral intake, the sodium and other electrolytes will need to be reduced accordingly to avoid progressively increasing IV fluid tonicity that can result in hypernatremia or other electrolyte derangements.
It is helpful to monitor the patient’s daily weight, urinary output, fluid input, and urine specific gravity. However, if stress-induced nonosmotic release of ADH is operative in a given patient, serial monitoring of the urine specific gravity may give the false impression that the child is still dehydrated when they are fluid replete, but still generating a concentrated urine. If fluid or electrolyte balance is abnormal, serial determination of serum electrolyte concentrations, blood urea nitrogen, and creatinine are necessary. In patients with significant burns, anuria, oliguria, or persistent abnormal stool or urine losses (eg, from a stoma, or polyuria secondary to a renal concentrating defect), it is important to measure output, and if needed its electrolyte components, so appropriate replacement can be provided.
DEHYDRATION
Depletion of body fluids is one of the most commonly encountered problems in clinical pediatrics. Children have a high incidence of gastrointestinal diseases, including gastroenteritis, and may demonstrate gastrointestinal symptoms in nongastrointestinal conditions, such as pneumonia or meningitis. Infants and young children often decrease their oral intake when ill, and their high ratio of surface area to weight promotes significant evaporative losses. Renal concentrating mechanisms do not maximally conserve water in early life, and fever may significantly increase fluid needs. Dehydration decreases ECF volume, leading to decreased tissue perfusion, progressive uremia and abnormal renal function studies, compensatory tachycardia, and lactic acidosis. The clinical effects of dehydration relate to the degree of dehydration and to the relative amounts of salt and water lost. Caregivers must be particularly aware of dehydration occurring in breast-fed newborn infants who go home soon after birth and whose mothers fail to produce enough milk. This problem is more common in the hot summer months and has been associated with severe dehydration, brain damage, and death.
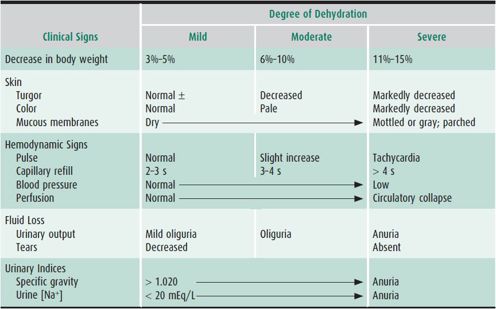
The clinical evaluation of a child with dehydration should focus on the composition and volume of fluid intake; the frequency and amount of vomiting, diarrhea, and urine output; the degree and duration of fever; the nature of any administered medications; and the existence of underlying medical conditions. A recently recorded weight, if known, can be very helpful in calculating the magnitude of dehydration. Important clinical features in estimating the degree of dehydration include the capillary refill time, postural blood pressure, and heart rate changes; dryness of the lips and mucous membranes; lack of tears; lack of external jugular venous filling when supine; a sunken fontanelle in an infant; oliguria; and altered mental status (Table 23–3). Children generally respond to a decrease in circulating volume with a compensatory increase in pulse rate and may maintain their blood pressure in the face of severe dehydration. A low or falling blood pressure is, therefore, a late sign of shock in children, and when present should prompt emergent treatment. Salient laboratory parameters include a high urine specific gravity (in the absence of an underlying renal concentrating defect as seen in diabetes insipidus or chronic obstructive or reflux nephropathy), a relatively greater elevation in blood urea nitrogen than in serum creatinine, a low urinary [Na+] excretion (< 20 mEq/L), and an elevated hematocrit or serum albumin level secondary to hemoconcentration.
Table 23–3. Clinical manifestations of dehydration.
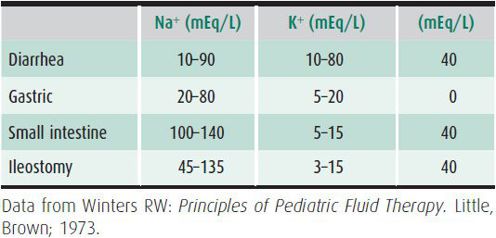
Emergent intravenous therapy is indicated when there is evidence of compromised perfusion (inadequate capillary refill, tachycardia, poor color, oliguria, or hypotension). The initial goal is to rapidly expand the plasma volume and to prevent circulatory collapse. A 20-mL/kg bolus of isotonic fluid should be given intravenously as rapidly as possible. Either colloid (5% albumin) or crystalloid (normal saline or Ringer lactate) may be used. Colloid is particularly useful in hypernatremic patients in shock, in malnourished infants, and in neonates. If no intravenous site is available, fluid may be administered intraosseously through the marrow space of the tibia. If there is no response to the first fluid bolus, a second bolus may be given. When adequate tissue perfusion is demonstrated by improved capillary refill, decreased pulse rate and urine output, and improved mental status, deficit replacement may be instituted. If adequate perfusion is not restored after 40 mL/kg of isotonic fluids, other pathologic processes must be considered such as sepsis, occult hemorrhage, or cardiogenic shock. Isotonic dehydration may be treated by providing half of the remaining fluid deficit over 8 hours and the second half over the ensuing 16 hours in the form of 5% dextrose with 0.45% saline containing 20 mEq/L of KCl. In the presence of metabolic acidosis, potassium acetate may be considered. Maintenance fluids and replacement of ongoing losses should also be provided. Typical electrolyte compositions of various body fluids are depicted in Table 23–4, although it may be necessary to measure the specific constituents of a patient’s fluid losses to guide therapy. If the patient is unable to eat for a prolonged period, nutritional needs must be met through hyperalimentation or enteral tube feedings.
Table 23–4. Typical electrolyte compositions of various body fluids.
Oral rehydration may be provided to children with mild to moderate dehydration. Clear liquid beverages found in the home, such as broth, soda, juice, and tea are inappropriate for the treatment of dehydration. Commercially available solutions provide 45–75 mEq/L of Na+, 20–25 mEq/L of K+, 30–34 mEq/L of citrate or bicarbonate, and 2%–2.5% of glucose. Frequent small aliquots (5–15 mL) should be given to provide approximately 50 mL/kg over 4 hours for mild dehydration and up to 100 mL/kg over 6 hours for moderate dehydration. Oral rehydration is contraindicated in children with altered levels of consciousness or respiratory distress who cannot drink freely; in children suspected of having an acute surgical abdomen; in infants with greater than 10% volume depletion; in children with hemodynamic instability; and in the setting of severe hyponatremia ([Na+] < 120 mEq/L) or hypernatremia ([Na+] > 160 mEq/L). Failure of oral rehydration due to persistent vomiting or inability to keep up with losses mandates intravenous therapy. Successful oral rehydration requires explicit instructions to caregivers and close clinical follow-up of the child.
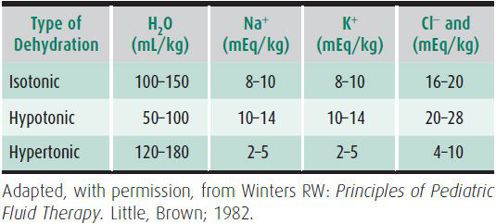
The type of dehydration is characterized by the serum [Na+]. If relatively more solute is lost than water, the [Na+] falls, and hyponatremic dehydration ([Na+] < 130 mEq/L) ensues. This is important clinically because hypotonicity of the plasma contributes to further volume loss from the ECF into the intracellular space. Thus, tissue perfusion is more significantly impaired for a given degree of hyponatremic dehydration than for a comparable degree of isotonic or hypertonic dehydration. It is important to note, however, that significant solute losses also occur in hypernatremic dehydration. Furthermore, because plasma volume is somewhat protected in hypernatremic dehydration, it poses the risk of the clinician underestimating the severity of dehydration. Typical fluid and electrolyte losses associated with each form of dehydration are shown in Table 23–5.
Table 23–5. Estimated water and electrolyte deficits in dehydration (moderate to severe).
HYPONATREMIA
Hyponatremia may be factitious in the presence of high plasma lipids or proteins, which decrease the percentage of plasma volume that is water. Hyponatremia in the absence of hypotonicity also occurs when an osmotically active solute, such as glucose or mannitol, is added to the ECF. Water drawn from the ICF dilutes the serum [Na+] despite isotonicity or hypertonicity.
Patients with hyponatremic dehydration generally demonstrate typical signs and symptoms of dehydration (see Table 23–3), because the vascular space is compromised as water leaves the ECF to maintain osmotic neutrality. The treatment of hyponatremic dehydration is fairly straightforward. The magnitude of the sodium deficit may be calculated by the following formula:
Na+ deficit = (Na+ desired − Na+ observed) × Bodyweight (kg) × 0.6
Half of the deficit is replenished in the first 8 hours of therapy, and the remainder is given over the following 16 hours. Maintenance and replacement fluids should also be provided. The deficit plus maintenance calculations often approximate 5% dextrose with 0.45% or higher saline. The rise in serum [Na+] should not exceed 0.5–1.0 mEq/L/h or 20 mEq/L/24 h unless the patient demonstrates central nervous system (CNS) symptoms that warrant more rapid initial correction. The dangers of too rapid correction of hyponatremia include cerebral dehydration and injury due to fluid shifts from the ICF compartment.
Hypovolemic hyponatremia also occurs in cerebral salt-wasting associated with CNS insults, a condition characterized by high urine output and elevated urinary [Na+] (> 80 mEq/L) due to an increase in ANF, and is a diagnosis of exclusion requiring a natriuresis in a patient with a contracted effective circulatory blood volume in the absence of other causes for Na+ excretion. This must be distinguished from the syndrome of inappropriate secretion of ADH (SIADH), which may also manifest in CNS conditions and pulmonary disorders. In contrast to cerebral salt-wasting, SIADH is characterized by euvolemia or mild volume expansion and relatively low urine output due to ADH-induced water retention. Urinary [Na+] is high in both conditions, though generally not as high as in SIADH. It is important to distinguish between these two conditions, because the treatment of the former involves replacement of urinary salt and water losses, whereas the treatment of SIADH involves water restriction. It is also important to remember that in SIADH patients are not necessarily oliguric, and that their urine does not need to be maximally concentrated but merely inappropriately concentrated for their degree of serum tonicity.
In cases of severe hyponatremia (serum [Na+] < 120 mEq/L) with CNS symptoms, intravenous 3% NaCl may be given over 1 hour to raise the [Na+] to 120 mEq/L to alleviate CNS manifestations and sequelae. In general, 6 mL/kg of 3% NaCl will raise the serum [Na+] by about 5 mEq/L. If 3% NaCl is administered, estimated Na+ and fluid deficits should be adjusted accordingly. Further correction should proceed slowly, as outlined earlier.
Hypervolemic hyponatremia may occur in edematous disorders such as nephrotic syndrome, congestive heart failure, and cirrhosis, wherein water is retained in excess of salt. Treatment involves restriction of Na+ and water and correction of the underlying disorder. Hypervolemic hyponatremia due to water intoxication is characterized by a maximally dilute urine (specific gravity < 1.003) and is also treated with water restriction.
HYPERNATREMIA
Although diarrhea is commonly associated with hyponatremic or isonatremic dehydration, hypernatremia may develop in the presence of persistent fever or decreased fluid intake or in response to improperly mixed rehydration solutions. Extreme care is required to treat hypernatremic dehydration appropriately. If the serum [Na+] falls precipitously, the osmolality of the ECF drops more rapidly than that of the CNS. Water shifts from the ECF compartment into the CNS to maintain osmotic neutrality. If hypertonicity is corrected too rapidly (a drop in [Na+] of > 0.5–1 mEq/L/h), cerebral edema, seizures, and CNS injury may occur. Thus, following the initial restoration of adequate tissue perfusion using isotonic fluids, a gradual decrease in serum [Na+] is desired (10–15 mEq/L/d). This is commonly achieved using 5% dextrose with 0.2% saline to replace the calculated fluid deficit over 48 hours. Maintenance and replacement fluids should also be provided. If the serum [Na+] is not correcting appropriately, the free water deficit may be estimated as 4 mL/kg of free water for each milliequivalent of serum [Na+] above 145 mEq/L and provided as 5% dextrose over 48 hours. If metabolic acidosis is also present, it must be corrected slowly to avoid CNS irritability. Potassium is provided as indicated—as the acetate salt if necessary. Electrolyte concentrations should be assessed every 2 hours in order to control the decline in serum [Na+]. Elevations of blood glucose and blood urea nitrogen may worsen the hyperosmolar state in hypernatremic dehydration and should also be monitored closely. Hyperglycemia is often associated with hypernatremic dehydration and may necessitate lower intravenous glucose concentrations (eg, 2.5%).
Patients with diabetes insipidus, whether nephrogenic or central in origin, are prone to develop profound hypernatremic dehydration as a result of unremitting urinary-free water losses (urine specific gravity < 1.010), particularly during superimposed gastrointestinal illnesses associated with vomiting or diarrhea. Treatment involves restoration of fluid and electrolyte deficits as described earlier as well as replacement of excessive water losses. Formal water deprivation testing to distinguish responsiveness to ADH should only be done during daylight hours after restoration of normal fluid volume status.
However, if a child presents with marked dehydration and a serum Na+ greater than 150 mEq/L, it may prove helpful and timely to obtain a plasma vasopressin level at the time of their initial presentation. The evaluation and treatment of nephrogenic and central diabetes insipidus are discussed in detail in Chapters 24 and 34, respectively.
Hypervolemic hypernatremia (salt poisoning), associated with excess total body salt and water, may occur as a consequence of providing improperly mixed formula, excessive NaCl or NaHCO3 administration, or as a feature of primary hyperaldosteronism. Treatment includes the use of diuretics, and potentially, concomitant water replacement or even dialysis.
POTASSIUM DISORDERS
The predominantly intracellular distribution of potassium is maintained by the actions of Na+-K+-ATPase in the cell membranes. Potassium is shifted into the ECF and plasma by acidemia and into the ICF in the setting of alkalosis, hypochloremia, or in conjunction with insulin-induced cellular glucose uptake. The ratio of intracellular to extracellular K+ is the major determinant of the cellular resting membrane potential and contributes to the action potential in neural and muscular tissue. Abnormalities of K+ balance are potentially life-threatening. In the kidney, K+ is filtered at the glomerulus, reabsorbed in the proximal tubule, and excreted in the distal tubule. Distal tubular K+ excretion is regulated primarily by the mineralocorticoid aldosterone. Renal K+ excretion is primarily dependent on the urinary flow rate, and continues for significant periods even after the intake of K+ is decreased. Thus, by the time urinary [K+] decreases, the systemic K+ pool has been depleted significantly. In general, the greater the urine flow the greater the urinary K+ excretion.
The causes of net K+ loss are primarily renal in origin. Gastrointestinal losses through nasogastric suction or vomiting reduce total body K+ to some degree. However, the resultant volume depletion results in an increase in plasma aldosterone, promoting renal excretion of K+ in exchange for Na+ reclamation to preserve circulatory volume. Diuretics (especially thiazides), mineralocorticoids, and intrinsic renal tubular diseases (eg, Bartter syndrome) enhance the renal excretion of K+. Systemic K+ depletion in hypokalemic metabolic acidosis may lead to “paradoxic aciduria” and low urine pH wherein H+ is preferentially exchanged for Na+ in response to aldosterone. Clinically, hypokalemia is associated with neuromuscular excitability, decreased peristalsis or ileus, hyporeflexia, paralysis, rhabdomyolysis, and arrhythmias. Electrocardiographic changes include flattened T waves, a shortened PR interval, and the appearance of U waves. Arrhythmias associated with hypokalemia include premature ventricular contractions; atrial, nodal, or ventricular tachycardia; and ventricular fibrillation. Hypokalemia increases responsiveness to digitalis and may precipitate overt digitalis toxicity. In the presence of arrhythmias, extreme muscle weakness, or respiratory compromise, intravenous K+ should be given. If the patient is hypophosphatemic ([PO43–]< 2 mg/dL), a phosphate salt may be used. The first priority in the treatment of hypokalemia is the restoration of an adequate serum [K+]. Providing maintenance amounts of K+ is usually sufficient; however, when the serum [K+] is dangerously low and K+ must be administered intravenously, it is imperative that the patient have a cardiac monitor. Intravenous K+ should generally not be given faster than at a rate of 0.3 mEq/kg/h. Oral K+ supplements may be needed for weeks to replenish depleted body stores.
Hyperkalemia—due to decreased renal K+ excretion, mineralocorticoid deficiency or unresponsiveness, or K+ release from the ICF compartment—is characterized by muscle weakness, paresthesias, and tetany; ascending paralysis; and arrhythmias. Electrocardiographic changes associated with hyperkalemia include peaked T waves, widening of the QRS complex, and arrhythmias such as sinus bradycardia or sinus arrest, atrioventricular block, nodal or idioventricular rhythms, and ventricular tachycardia or fibrillation. The severity of hyperkalemia depends on the electrocardiographic changes, the status of the other electrolytes, and the stability of the underlying disorder. A rhythm strip should be obtained when significant hyperkalemia is suspected. If the serum [K+] is less than 6.5 mEq/L, discontinuing K+ supplementation may be sufficient if there is no ongoing K+ source, such as cell lysis, and if urine output continues. If the serum [K+] is greater than 7 mEq/L or if potentiating factors such as hyponatremia, digitalis toxicity, and renal failure are present, more aggressive therapy is needed. If electrocardiographic changes or arrhythmias are present, treatment must be initiated promptly. Intravenous 10% calcium gluconate (0.2–0.5 mL/kg over 2–10 minutes) will rapidly ameliorate depolarization and may be repeated after 5 minutes if electrocardiographic changes persist. Calcium should be given only with a cardiac monitor in place and should be discontinued if bradycardia develops. The intravenous administration of a diuretic that acts in the loop of Henle, such as furosemide (1–2 mg/kg), will augment renal K+ excretion and can be very helpful in lowering serum and total body [K+]. Administering Na+ and increasing systemic pH with bicarbonate therapy (1–2 mEq/kg) will shift K+ from the ECF to the ICF compartment, as will therapy with a β-agonist such as albuterol. In nondiabetic patients, 0.5 g/kg of glucose over 1–2 hours will enhance endogenous insulin secretion, lowering serum [K+] 1–2 mEq/L. Administration of intravenous glucose and insulin may be needed as a simultaneous drip (0.5–1 g/kg glucose and 0.3 units of regular insulin per gram of glucose) given over 2 hours with monitoring of the serum glucose level every 15 minutes.
The therapies outlined above provide transient benefits. Ultimately, K+ must be reduced to normal levels by reestablishing adequate renal excretion using diuretics or optimizing urinary flow, using ion exchange resins such as sodium polystyrene sulfonate orally or as a retention enema (0.2–0.5 g/kg orally or 1 g/kg as an enema), or by dialysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


