Methods
Search strategy
This metaanalysis was performed according to a protocol recommended for systematic review. The review protocol was designed a priori to define methods for collecting, extracting, and analyzing data. The research was conducted with the use of MEDLINE, EMBASE, Web of Sciences, Scopus, ClinicalTrial.gov , OVID, and Cochrane Library as electronic databases. The trials were identified with the use of a combination of the following text words: “fetal fibronectin,” “preterm labor,” “threatened,” “prediction,” “prevention,” “birth,” “delivery,” “prematurity,” “neonatal,” and “randomized” from the inception of each database to February 2016. Review of articles also included the abstracts of all references that were retrieved from the search.
Study selection
Selection criteria included RCTs of singleton gestations with threatened PTL that were assigned randomly to management based on FFN results (ie, intervention group) or not (ie, comparison group). We included both studies in which FFN was collected on all women and studies in which FFN screening was done only on women who were assigned randomly to the FFN group. In the studies in which FFN was collected on all women, women were assigned randomly so that, in 50% of them, the result was available to them and the managing obstetrician, and, in 50% of them, the FFN was blinded to them and the managing obstetricians. Types of participants included women with singleton gestations at 23 0/7 to 34 6/7 weeks of gestation with threatened PTL.
Studies that included management that was based also on the use of sonographic cervical length were excluded. Quasirandomized trials (ie, trials in which allocation was done on the basis of a pseudo-random sequence [eg, odd/even hospital number or date of birth] alternation) and studies on multiple pregnancies were also excluded.
Data extraction and risk of bias assessment
The risk of bias in each included study was assessed by the use of the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions . Seven domains that are related to risk of bias were assessed in each included trial because there is evidence that these issues are associated with biased estimates of treatment effect: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. Review authors’ judgments were categorized as “low risk,” “high risk,” or “unclear risk” of bias.
All analyses were done with an intention-to-treat approach; conditions were evaluated according to the treatment group to which they were allocated randomly in the original trials. The primary outcome was the incidence of PTB <37weeks. Secondary maternal outcomes were PTB at <34, <32, and <28 weeks of gestation, delivery within 7 days, mean gestational age at delivery (in weeks), maternal hospitalization, tocolysis, use of antenatal steroids, mean time to evaluate (in hours), neonatal outcomes (ie, incidence of respiratory distress syndrome and of admission to neonatal intensive care unit) and hospitalization charges. Data from each eligible study were extracted without modification of original data onto custom-made data collection forms. Data not present in the original publications were requested from all the principal investigators.
Data analysis
The data analysis was completed independently by the authors who used Review Manager (version 5.3; The Nordic Cochrane Centre, Cochrane Collaboration, 2014, Copenhagen, Denmark). The completed analyses were then compared, and any difference was resolved with review of the entire data and independent analysis. Between-study heterogeneity was explored with the I 2 statistic, which represents the percentage of between-study variation that is due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, whereas I 2 values of ≥50% indicate a substantial level of heterogeneity. A fixed effects model was used if substantial statistical heterogeneity was not present. On the contrary, if there was evidence of significant heterogeneity between the studies that were included, a random effect model was used.
Potential publication biases were assessed statistically with Begg’s and Egger’s tests. A probability value of <.05 was considered statistically significant. Tests for funnel plot asymmetry were carried out with only an exploratory aim when the total number of publications that were included for each outcome was <10. In this case, the power of the tests was too low to distinguish chance from real asymmetry.
The summary measures were reported as relative risk (RR) or as mean difference (MD) with 95% confidence interval (CI).
All review stages were conducted independently by the authors who independently assessed electronic search, eligibility of the studies, inclusion criteria, risk of bias, data extraction, and data analysis. Disagreements were resolved by discussion.
The metaanalysis was reported according to the Preferred Reporting Item for Systematic Reviews and Meta-analyses (PRISMA) statement. Before data extraction, the review was registered with the PROSPERO International Prospective Register of Systematic Reviews (registration No.: CRD42016035939).
Results
Study selection and study characteristics
Figure 1 shows the flow diagram (PRISMA template) of information derived from the review of potentially relevant articles. Eight studies were assessed for eligibility. Two studies were excluded : one study was excluded because it assessed the efficacy of FFN in women with previous SPTB ; Ness et al was excluded because the management was based also on the use of ultrasound cervical length. Six trials therefore were included in the metaanalysis. Tests for funnel plot asymmetry were carried out only with an exploratory aim because the total number of publications that were included for each outcome was <10. Despite this, the overall risk of bias of the included trials was low ( Figure 2 ). All studies had a low risk of bias in “random sequence generation,” “incomplete outcome data,” and “selective reporting.” Adequate methods for allocation of women were used. All randomly assigned women were included in an intention-to-treat analysis. In 3 studies, laboratory personnel who performed the FFN test were blinded to women’s characteristics and outcomes (ie, blinding of outcome assessment). Physicians were not blinded to the FFN assay result. Publication bias, which was assessed with the use of Begg’s and Egger’s tests, was not significant ( P = .84 and .91, respectively). Authors of 4 trials were able to provide us additional unpublished data from their studies.
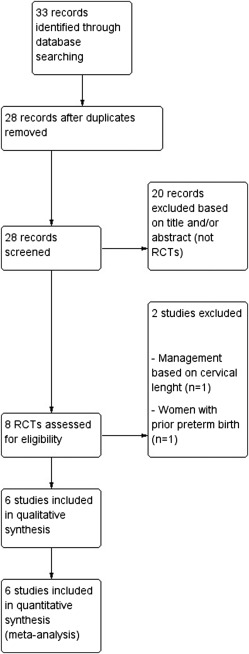
Table 1 shows the characteristics of the included trials. Women were eligible for the randomization in case of symptoms that suggested PTL between 23 and 34 weeks of gestation. During admission, before digital examination, a Dacron swab was rotated in the posterior fornix for 10 seconds to absorb cervicovaginal secretions that were then analyzed for the FFN qualitative method, with results reported as either positive or negative. Women with cervical manipulation or sexual intercourse within the previous 24 hours, ruptured membranes, gross bleeding, cervical dilation ≥3 cm, cerclage in situ, and multiple pregnancies were excluded in all the trials. The treatment of the women was mostly at the physician’s discretion ( Table 2 ).
| Study location | Nguyen et al, 2002 | Plaut et al, 2003 | Grobman et al, 2004 | Lowe et al, 2004 | Dutta and Norman, 2011 | Lee et al, 2013 |
|---|---|---|---|---|---|---|
| United States | United States and Canada | United States | United States | United Kingdom | United States | |
| Sample size a | 77 (42 vs 35) | 108 (51 vs 57) | 100 (50 vs 50) | 97 (46 vs 51) | 88 (44 vs 44) | 76 (44 vs 32) |
| Inclusion criteria | Singleton gestations with symptoms of preterm labor | Singleton gestations with symptoms of preterm labor | Singleton gestations with symptoms of preterm labor | Singleton gestations with symptoms of preterm labor | Singleton gestations with symptoms of preterm labor | Singleton gestations with symptoms of preterm labor |
| Definition of preterm labor | Uterine contractions, low back pain, or bloody show b | Not reported | >6 Contractions per hour by external tocodynometry | Not reported | Uterine contractions, low back pain, pelvic pressure, or low abdominal pressure | >3 Contractions per 30 minutes by external tocodynometry, abdominal pressure or cramping, low back pain |
| Gestational age at randomization (range in weeks) | 24 0 –34 6 | 24 0 –34 6 | 24 0 –34 6 | 24 0 –34 6 | 24 0 –34 6 | 24 0 –33 6 |
| Exclusion criteria | Cervical manipulation or sexual intercourse within the previous 24 hours, ruptured membranes, gross bleeding, cervical dilation ≥3 cm, cerclage in situ, multiple pregnancies | Cervical manipulation or sexual intercourse within the previous 24 hours, ruptured membranes, gross bleeding, cervical dilation ≥3 cm, cerclage in situ, multiple pregnancies | Cervical manipulation or sexual intercourse within the previous 24 hours, ruptured membranes, gross bleeding, cervical dilation ≥3 cm, cerclage in situ, multiple pregnancies | Cervical manipulation or sexual intercourse within the previous 24 hours, ruptured membranes, gross bleeding, cervical dilation ≥3 cm, cerclage in situ, multiple pregnancies | Cervical manipulation or sexual intercourse within the previous 24 hours, ruptured membranes, gross bleeding, cervical dilation ≥3 cm, cerclage in situ, multiple pregnancies | Cervical manipulation or sexual intercourse within the previous 24 hours, ruptured membranes, gross bleeding, cervical dilation ≥3 cm, cerclage in situ, multiple pregnancies |
| Fetal fibronectin immunoassay test | Adeza Biomedical | Adeza Biomedical | Adeza Biomedical | Adeza Biomedical | Not reported | Adeza Biomedical |
| Fetal fibronectin cut-off | 50 ng/mL | 50 ng/mL | 50 ng/mL | 50 ng/mL | 50 ng/mL | 50 ng/mL |
| Control group | Fetal fibronectin not done | Fetal fibronectin blinded | Fetal fibronectin blinded | Fetal fibronectin blinded | Fetal fibronectin blinded | Fetal fibronectin blinded |
| Previous spontaneous preterm birth a | 11/42 (26.2%) vs 8/35 (22.9%) b | 17/51 (33.3%) vs 25/57 (43.9) | 4/50 (8.0%) vs 8/50 (16.0%) | 12/46 (26.1%) vs 14/51 (27.5%) | Not reported | 12/44 (27.3%) vs 9/32 (28.1%) |
| Primary outcome | Cost-effectiveness | Transport to tertiary care centers | Health care costs | Length of stay | Inpatient hospital admission | Triage evaluation time |
a Data are presented as number in the intervention group (ie, fetal fibronectin group) vs number in the control group
b Additional data provided by the authors of the original trial.
| Variable | Nguyen et al, 2002 | Plaut et al, 2003 | Grobman et al, 2004 | Lowe et al, 2004 | Dutta and Norman, 2011 | Lee et al, 2013 |
|---|---|---|---|---|---|---|
| Fetal fibronectin positive | Further observation | Physician’s discretion a | Physician’s discretion a | Physician’s discretion a | Admit for preterm labor, administration of tocolytics and steroids | Admit for preterm labor b or discharge |
| Fetal fibronectin negative | Discharged home | Physician’s discretion a | Physician’s discretion a | Physician’s discretion a | Discharged home | Discharged home |
| Fetal fibronectin blinded or not done | Physician’s discretion | Physician’s discretion | Physician’s discretion | Physician’s discretion | Physician’s discretion | Physician’s discretion |
a Decision made by attending physician who was aware of the test results and test characteristics (sensitivity, specificity, and positive and negative predictive value) of the fetal fibronectin assay
b If cervical change in serial cervical examinations or if persistent contractions.
Synthesis of results
Table 3 shows the pooled results for the primary and secondary outcomes. Of the 546 singleton gestations with symptoms of PTL that were included in the metaanalysis, 277 gestations (50.7%) were assigned randomly to the FFN group, and 269 gestations (49.3%) were assigned to the comparison group. Statistical heterogeneity was low with no inconsistency (I 2 = 0) in the primary outcome. Compared with control group, women who were assigned randomly to FFN group had a similar incidence of PTB at <37 weeks of gestation (20.7% vs 29.2%; RR, 0.72; 95% CI, 0.52–1.01; Figure 3 ), <34 weeks of gestation (8.3% vs 7.9%; RR, 1.09; 95% CI, 0.54–2.18), <32 weeks of gestation (3.3% vs 5.6%; RR, 0.64; 95% CI, 0.24–1.74), and <28 weeks of gestation (1.1% vs 1.7%; RR, 0.74; 95% CI, 0.15–3.67). No differences were found in the number of women who delivered within 7 days (12.8% vs 14.5%; RR, 0.76; 95% CI, 0.47–1.21), in the mean of gestational age at delivery (MD 0.20 week; 95% CI, –0.26–0.67; Figure 4 ), in the rate of maternal hospitalization (27.4% vs 26.9%; RR, 1.07; 95% CI, 0.80–1.44), in use of tocolysis (25.3% vs 28.2%; RR, 0.97; 95% CI, 0.75–1.24; Figure 5 ), antenatal steroids (29.2% vs 29.2%; RR, 1.05; 95% CI, 0.79–1.39), in the mean time in the triage unit (MD, 0.60 hour; 95% CI, –0.03–1.23), and in neonatal outcomes that included respiratory distress syndrome (1.3% vs 1.5%; RR, 0.91; 95% CI, 0.06–14.06) and admission to neonatal intensive care unit (19.4% vs 8.1%; RR, 2.48; 95% CI, 0.96–6.46). Management based on FFN testing required higher hospitalization charges (MD, $153; 95% CI, 24.01–281.99).
| Variable | Nguyen et al, 2002 | Plaut et al, 2003 | Grobman et al, 2004 | Lowe et al, 2004 | Dutta and Norman, 2011 | Lee et al, 2013 | Total | Relative risk (95% confidence interval) |
|---|---|---|---|---|---|---|---|---|
| Sample size a | 77 (42 vs 35) | 108 (51 vs 57) | 100 (50 vs 50) | 97 (46 vs 51) | 88 (44 vs 44) | 76 (44 vs 32) | 546 (277 vs 269) | — |
| Preterm birth a | ||||||||
| At <37 weeks of gestation | 11/42 (26.2%) vs 9/35 (25.7%) b | 5/43 (11.6%) vs 12/47 (25.5%) | 10/50 (20.0%) vs 13/50 (26.0%) | 13/46 (28.3%) vs 24/51 (47.1%) b | Not reported | 7/41 (17.1%) vs 4/29 (13.8%) | 46/222 (20.7%) vs 62/212 (29.2%) | 0.72 (0.52–1.01) |
| At <34 weeks of gestation | Not reported | 2/43 (4.7%) vs 2/47 (4.3%) | 5/50 (10.0%) vs 3/50 (6.0%) | 5/46 (10.9%) vs 9/51 (17.7%) b | Not reported | 3/41 (7.3%) vs 0/29 | 15/180 (8.3%) vs 14/177 (7.9%) | 1.09 (0.54–2.18) |
| At <32 weeks of gestation | Not reported | 2/43 (4.7%) vs 1/47 (2.1%) | 3/50 (6.0%) vs 2/50 (4.0%) | 1/46 (2.1%) vs 7/51 (13.7%) b | Not reported | 0/41 vs 0/29 b | 6/180 (3.3%) vs 10/177 (5.6%) | 0.64 (0.24–1.74) |
| At <28 weeks of gestation | Not reported | 0/43 vs 0/47 | 2/50 (4.0%) vs 2/50 (4.0%) | 0/46 vs 1/51 (1.9%) b | Not reported | 0/41 vs 0/29 b | 2/180 (1.1%) vs 3/177 (1.7%) | 0.74 (0.15–3.67) |
| Delivery within 7 days a | 1/42 (2.4%) vs 2/35 (5.7%) b | Not reported | 2/50 (4.0%) vs 3/50 (6.0%) b | 3/46 (6.5%) vs 4/51 (7.8%) b | Not reported | 17/41 (41.5%) vs 15/29 (51.7%) b | 23/179 (12.8%) vs 24/165 (14.5%) | 0.76 (0.47–1.21) |
| Gestational age at delivery, wk c | 34.2±2.9 vs 33.7±2.7 b | 29.9±3.2 vs 30.4±2.7 | 38±3 vs 38±3 | 38.3±2.8 vs 37.4±3.4 | 38.1±3.25 vs 38.1±2.33 | 38.6±2.1 vs 38.3±1.7 b | — | 0.20 week (–0.26–0.67) |
| Maternal hospitalization a | 9/42 (21.4%) vs 7/35 (20.0%) b | Not reported | 13/50 (26.0%) vs 14/50 (28.0%) | 16/46 (34.8%) vs 12/51 (23.5%) | 21/44 (47.7%) vs 22/44 (50.0%) | 3/44 (6.8%) vs 2/32 (6.3%) | 62/226 (27.4%) vs 57/212 (26.9%) | 1.07 (0.80–1.44) |
| Tocolysis a | 7/42 (16.7%) vs 7/35 (20.0%) b | 25/43 (58.1%) vs 28/47 (59.6%) | 8/50 (16.0%) vs 9/50 (18.0%) | 22/46 (47.8%) vs 23/51 (45.1%) | 3/44 (6.8%) vs 4/44 (9.1%) | 3/44 (6.8%) vs 2/32 (6.3%) b | 68/269 (25.3%) vs 73/259 (28.2%) | 0.97 (0.75–1.24) |
| Steroids a | 9/42 (21.4%) vs 7/35 (20.0%) b | Not reported | 8/50 (16.0%) vs 10/50 (20.0%) | 23/46 (50.0%) vs 22/51 (43.1%) | 17/44 (38.6%) vs 21/44 (47.7%) | 9/44 (20.5%) vs 2/32 (6.3%) b | 66/226 (29.2%) vs 62/212 (29.2%) | 1.05 (0.79–1.39) |
| Time in the triage unit, hr c , d | 3.3±1.7 vs 2.7±1.7 | 6.3±8 vs 10±28.1 | 4.12±3.59 vs 4.49±3.90 b | 16±7.4 vs 12±4.9 | 16.8±25.3 vs 17.7±25.5 | 3±1.8 vs 2.8±1.6 | — | 0.60 hour (–0.03–1.23) |
| Respiratory distress syndrome a | Not reported | Not reported | Not reported | Not reported | 1/44 (2.3%) vs 1/40 (2.5%) | 0/37 vs 0/27 b | 1/81 (1.3%) vs 1/67 (1.5%) | 0.91 (0.06–14.06) |
| Admission to neonatal intensive care unit a | Not reported | Not reported | Not reported | Not reported | 10/30 (33.3%) vs 3/30 (10.0%) | 3/37 (8.1%) vs 2/32 (6.3%) | 13/67 (19.4%) vs 5/62 (8.1%) b | 2.48 (0.96–6.46) |
| Hospitalization charges, $ c | 452±381 vs 299±175 | Not reported | Not reported | Not reported | Not reported | Not reported | — | $153 (24.01–281.99) e |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


