Objective
Studies have questioned the long-term health effects of offspring conceived after fertility treatments.
Methods
We aimed to evaluate whether an association exists between mode of conception (in vitro fertilization, ovulation induction, or spontaneous pregnancy) and neoplasm risk (both benign and malignant tumors) among the offspring; we observed the offspring for up to 18 years.
Study Design
A population-based cohort analysis was performed that compared the risk for neoplasms among children (up to the age of 18 years) based on mode of conception. Neoplasm diagnoses were based on hospital records of the same single tertiary center in the region. All singletons born during from 1991–2013 and discharged alive were included in the study. Offspring with congenital malformations were excluded from the analysis. Kaplan-Meier survival curves were constructed to compare cumulative neoplasms incidence; multivariable survival analyses were used to control for confounders that included gestational age, pregnancy complications, and maternal factors.
Results
During the study period, 242,187 newborn infants met the inclusion criteria: 2603 (1.1%) were conceived after in vitro fertilization; 1721 (0.7%) were conceived after ovulation induction treatments, and 237,863 (98.3%) were conceived spontaneously. During the follow-up period (median, 10.55 years), 1498 neoplasms(0.6%) were diagnosed. Incidence density rate for neoplasms was higher among children conceived either after in vitro fertilization (1.5/1000 person years) or ovulation induction treatments (1.0/1000 person years), as compared with naturally conceived children (0.59/1000 person years; Kaplan-Meier log rank, P <.001). The association between in vitro fertilization and total pediatric neoplasms and the association between any fertility treatments and malignancies remained significant; we controlled for confounders such as gestational diabetes mellitus, hypertensive disorders, preterm birth, and maternal age (adjusted hazard ratio, 2.48; 95% confidence interval, 1.71–3.50; and adjusted hazard ratio, 1.96; 95% confidence interval, 1.14–3.36, for all neoplasms and all malignancies, respectively).
Conclusion
Children conceived after fertility treatments are at an increased risk for pediatric neoplasms.
Infertility, which affects approximately 9% of the population, is considered a disease, and can be treated with several modalities. Treatment may include ovulation induction (OI), in vitro fertilization (IVF), surgery, or a combination of several options. OI is used in cases of anovulation, unexplained infertility, or mild male factor infertility. It may be used as a sole treatment modality or as a preparation for intrauterine insemination. IVF alone or combined with additional manipulations of either the egg or the sperm is considered assisted reproduction technologies (ART). The first “test tube baby” was born in the United Kingdom in 1978 as a result of IVF; since then, ART has grown in popularity and success rates. In 2010, live birth rates that resulted from ART accounted for 1–4% of all deliveries in the Western world. Fertility treatments largely have been investigated regarding adverse pregnancy outcomes and have been found to be associated with an increased risk of neonatal death, low birthweight, preterm birth, and congenital malformations. These adverse outcomes may be due to the infertility or subfertility cause that leads to ART or due to the manipulation itself.
Fertility treatments have also been studied in association with offspring health outcomes throughout life, which includes cancer risk. Several mechanisms were suggested as underlying this association. Interrupted gene regulation and tumor suppression, overcoming the natural selection and survival mechanism present in spontaneous pregnancies, thus leaving embryos with higher risk for abnormalities that include birth defects and neoplasms. Epigenetic changes that are associated with ART or the infertility itself may impact the future risk of neoplasms because of imprinting disorders, altered gene expressions, and methylation levels. Finally, the exogenous hormone administration may affect the fetus during the critical period of growth and differentiation, thereby increasing endocrine-sensitive cancer risk later in life. Certain cancers develop from benign tumors; thus, causes and risk factors for benign and malignant tumors may be similar or interrelated.
Fertility treatments, and specifically ART, are relatively new exposures; therefore, much is yet to be elucidated regarding the possible long-term health effects among offspring conceived by these treatments. Because the numbers of offspring who were exposed to fertility treatments are rising and because offspring age, it is critical to understand the possible health impact of such exposure and specifically the risk for future pediatric neoplasms. The aim of this study was to study the association between mode of conception (IVF, OI, or spontaneous pregnancy) and neoplasm risk among the offspring, followed for up to 18 years.
Methods
A population-based cohort analysis was performed that included all singleton infants born during the years 1991–2013 and discharged alive from Soroka University Medical Center (SUMC). SUMC includes the largest birth center in Israel and is the only tertiary hospital in Israel’s southern region and the single IVF unit in the region. In Israel all fertility interventions, which includes IVF, are covered fully by a national health law (1997), which allows citizens of all backgrounds access to these treatments.
The study protocol was approved by the SUMC institutional review board, and informed consent was exempt.
The independent variable was defined as mode of conception: spontaneous vs after OI or IVF treatments. OI included the use of an oral agent or gonadotropins, oocyte trigger for final maturation with human chorionic gonadotropin, combined with intrauterine insemination or an intercourse according to semen parameters. IVF included procedures that involved oocyte retrieval and manipulation of the egg and the sperm. Mode of conception was reported by the parturient and verified through the prenatal medical records, which was summarized into the electronic chart.
The perinatal dataset consists of information that was recorded directly after delivery by an obstetrician. Skilled medical secretaries routinely reviewed the information before entering it into the database. Coding was performed after assessment of medical prenatal care records together with routine hospital documents. This perinatal dataset was cross-linked and matched with the pediatric hospitalization dataset, based on personal identifying numbers.
The outcome variable was defined as the first pediatric hospitalization of the offspring with any neoplasm diagnosis. Neoplasm diagnoses reflect both benign and malignant tumors. The pediatric hospitalization dataset includes International Classification of Diseases, 9th revision, codes for all medical diagnoses, as well as demographic information. All neoplasm diagnoses that were mentioned in the pediatric admission/discharge database were identified and grouped by a certified pediatrician (I.S.) according to systems and organs. A list of the grouped diagnoses and International Classification of Diseases, 9th revision, codes is presented in the Appendix . A neoplasm event was defined as the first hospitalization with any diagnoses from the neoplasm codes list. Follow-up time in the study was calculated from birth to an event, non-neoplasm–related death (if occurred in the hospital), or end of the study period or age 18 year. We assumed loss of follow up was not related to exposure and outcome and therefore no representation bias occurred. If >1 neoplasm diagnosis existed in the first hospitalization, all diagnoses were counted and compared between the study groups, in both univariable and multivariable levels. In the “all neoplasm” multivariable analysis, only 1 diagnosis was included per child: the malignant diagnosis or the first benign diagnosis in case no malignant diagnosis was present.
To reduce potential confounders, offspring with congenital malformations or multiple gestations were excluded from the study. To address the possible changes in fertility treatments over the years, a categoric time variable was created, with each category representing a 2-year interval. The effect of this variable has been tested in the multivariable model.
Initial analysis compared background, pregnancy, and perinatal characteristics of the 3 study groups, with the use of chi square, analysis of variance, and Kruskal-Wallis tests, based on variable characteristics and normal distribution. All analyses were 2-sided; a probability value of <.05 was considered statistically significant.
Background and perinatal characteristics included maternal age, ethnicity, parity, pregnancy complications (pregestational or gestational diabetes mellitus [GDM]; chronic or hypertensive disorders of pregnancy, which include eclampsia and preeclampsia), gestational age at delivery, and offspring gender.
Outcome variables included any neoplasm, malignant or benign, diagnoses during hospitalization of the offspring, as defined in the Appendix . Incidence rates per 10,000 offspring and incidence density rates per 1000 person years (PY), which adjusted for differences in follow-up time between the study groups, were calculated and compared between study groups. Kaplan-Meier survival curves were constructed, and the cumulative neoplasms and malignancy hospitalization incidence were compared between the groups with the use of the Cox-Mantel log rank test.
Variables that were statistically different between the study groups and that were associated with neoplasm risks that were suspected as confounders or variables with clinical importance were considered in the multivariable models. To adjust for length of follow up, a multivariable Weibull parametric survival analysis was performed, in which mothers in the cohort were entered as clusters and the dependence among the siblings was accounted for. The models compared the independent risk for total neoplasms in the offspring, total malignancies or benign neoplasms, based on mode of conception while adjusting for confounders.
Results
During the study period, 242 187 newborn infants met the inclusion criteria: 2603 of whom (1.1%) were conceived after IVF; 1172 of whom (0.7%) were conceived after OI, and 237,863 of whom (98.3%) were with pregnancies that were conceived spontaneously. The rates of IVF have changed from 0.2% of the offspring in 1991 to 1.8–2.0% in the years 2012–2013; OI rates were 1.0% (1991) and 0.5% (2013). Table 1 presents background information of the study population, which includes demographic, obstetric, and perinatal characteristics. Mothers in the 3 study groups were different in terms of age, with IVF mothers being older than OI mothers, and both were older than the comparison group. In addition, both fertility treatment groups exhibited lower parity and gravidity, shorter mean gestation, and higher rates of preterm births and low birthweights. Pre-GDM and hypertension and GDM and hypertensive disorders were more prevalent among the fertility treatment groups. Mean follow-up time also differed by study group, with the longest follow-up time in the OI group.
| Characteristic | In vitro fertilization (n=2603; 1.1%) | Ovulation induction (n=1721; 0.7%) | Spontaneous (n=237,863; 98.3%) | P value |
|---|---|---|---|---|
| Maternal age at delivery, y a | 32.51±5.38 | 29.54±5.22 | 28.10±5.81 | <.001 |
| Gestational age, wk a | 38.25±1.89 | 38.74±1.96 | 39.16±1.74 | <.001 |
| Parity, n (%) | <.001 | |||
| 1 | 1355 (52.1) | 987 (57.4) | 54,790 (23.0) | |
| 2-4 | 1211 (46.5) | 697 (40.5) | 121,962 (51.3) | |
| ≥5 | 36 (1.4) | 37 (2.1) | 61,076 (25.7) | |
| Newborn gender (male), n (%) | 1288 (49.5) | 854 (49.6) | 120,973 (50.9) | .23 |
| Preterm delivery (gestational week <37), n (%) | 326 (12.5) | 161 (9.4) | 15,218 (6.4) | <.001 |
| Low birthweight (<2500 g), n (%) | 290 (11.1) | 196 (11.4) | 149,605 (6.3) | <.001 |
| Very low birthweight (<1500 g), n (%) | 25 (1.0) | 16 (0.9) | 759 (0.3) | <.001 |
| Small for gestational age, n (%) | 116 (4.5) | 118 (6.9) | 10,764 (4.5) | <.001 |
| Low Apgar 5 score (<7), n (%) | 21 (0.8) | 12 (0.7) | 4,274 (1.8) | <.001 |
| Mode of delivery, n (%) | <.001 | |||
| Cesarean | 1016 (39.0) | 445 (25.9) | 31,267 (13.1) | |
| Assisted | 118 (4.5) | 81 (4.7) | 7,590 (3.2) | |
| Vaginal | 1469 (56.4) | 1195 (69.4) | 199,006 (83.7) | |
| Pregestational diabetes mellitus, n (%) | 82 (3.2) | 53 (3.1) | 2,348 (1.0) | <.001 |
| Gestational diabetes mellitus, n (%) | 289 (11.1) | 171 (9.9) | 9,219 (3.9) | <.001 |
| Pregestational hypertension, n (%) | 89 (3.4) | 74 (4.3) | 3,105 (1.3) | <.001 |
| Gestational hypertensive disorders, n (%) | 265 (10.2) | 204 (11.9) | 11,638 (4.9) | <.001 |
| Follow-up time, d a | 2790.85±2028.9 | 4224.7±2147.0 | 3767.12±2157.43 | <.001 |
During the follow-up period (median, 10.55 years; range, 0–18 years), 1498 neoplasms (0.6% of the entire study population) were diagnosed. Rates per 10,000 offspring by neoplasm category are presented in Table 2 . The rate (per 10,000) of malignancies was highest in the OI group; benign tumors were most prevalent in the IVF group, and rates of total neoplasm morbidities were similar in the IVF and OI groups and were significantly higher than in the spontaneously conceived group. The incidence density rates (per 1000 person year of follow up, which controlled at the univariable level for the different lengths of follow up) for malignancies were higher in either IVF or OI groups compared with the spontaneously conceived offspring (data not presented): 0.4/1000 PY in either IVF or OI, and 0.17/1000 PY among the spontaneously conceived children. For benign morbidity and for total neoplasm morbidities, the highest incidence density rate was among the IVF group, followed by the OI group; the lowest rates were among the spontaneously conceived offspring (benign: 1.1/1000 PY, 0.6/1000 PY, and 0.42/1000 PY among the IVF, OI, and spontaneous groups, respectively; total neoplasms: 1.5/1000 PY, 1.0/1000 PY, and 0.59/1000 PY among the IVF, OI and spontaneous groups, respectively).
| Variable | Total (N=242,187; 100%) | In vitro fertilization (n=2603; 1.1%) | Ovulation induction (n=1721; 0.7%) | Spontaneous (n=237,863; 98.3%) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Rate per 10,000 | n | Rate per 10,000 | n | Rate per 10,000 | n | Rate per 10,000 | ||
| Total malignancies | 429 | 17.71 | 7 | 26.89 | 7 | 40.67 | 415 | 17.44 | .038 |
| Total benign | 1074 | 44.35 | 22 | 84.51 | 12 | 69.73 | 1040 | 43.72 | .002 |
| Total neoplasm | 1498 | 61.85 | 29 | 111.41 | 19 | 110.40 | 1450 a | 60.96 | <.001 |
| Malignancies | |||||||||
| Head and neck | 12 | 0.50 | 0 | — | 1 | 5.81 | 11 | 0.46 | .007 |
| Peritoneum/ retroperitoneum | 3 | 0.12 | 0 | 0 | 0 | — | 3 | 0.13 | .98 |
| Lung | 3 | 0.12 | 0 | — | 0 | — | 3 | 0.13 | .97 |
| Mediastinum | 2 | 0.08 | 0 | — | 0 | — | 2 | 0.08 | .98 |
| Bone | 12 | 0.50 | 0 | — | 2 | 11.62 | 10 | 0.42 | <.01 |
| Connective tissue | 12 | 0.50 | 0 | — | 0 | — | 12 | 0.50 | .90 |
| Skin | 4 | 0.17 | 0 | — | 0 | — | 4 | 0.17 | .97 |
| Vagina/ vulva / testes | 7 | 0.29 | 0 | — | 0 | — | 7 | 0.29 | .95 |
| Kidney | 11 | 0.45 | 0 | — | 0 | — | 11 | 0.46 | .91 |
| Ophthalmic | 4 | 0.17 | 0 | — | 1 | 5.81 | 3 | 0.13 | <.01 |
| Brain | 38 | 1.57 | 1 | 3.84 | 1 | 5.81 | 36 | 1.51 | .24 |
| Adrenal | 7 | 0.29 | 0 | — | 0 | — | 7 | 0.29 | .94 |
| Secondary | 7 | 0.29 | 0 | — | 1 | 5.81 | 6 | 0.25 | .006 |
| Lymphoma | 55 | 2.27 | 2 | 7.68 | 0 | — | 53 | 2.23 | .15 |
| Leukemia | 90 | 3.72 | 0 | — | 1 | 5.81 | 89 | 3.74 | .56 |
| Hemangioma | 139 | 5.74 | 3 | 11.52 | 1 | 5.81 | 135 | 5.68 | .46 |
a Among the spontaneously conceived children, 5 were diagnosed with both benign and malignant morbidities.
Figure 1 presents the Kaplan-Meier log of survival for total neoplasms by study group. The differences between the groups were significant (Log rank, P <.001).The post hoc tests revealed that the differences in survival were significant only between the spontaneously conceived group compared with the IVF group and between the OI and IVF groups. Figure 2 presents the Kaplan-Meier log of survival for malignant neoplasms only. Here again, the differences between the groups were statistically significant (Log rank, P =.023). The post hoc tests revealed that there was no difference between the IVF and OI groups, but between either OI and IVF as compared with the spontaneously conceived group.
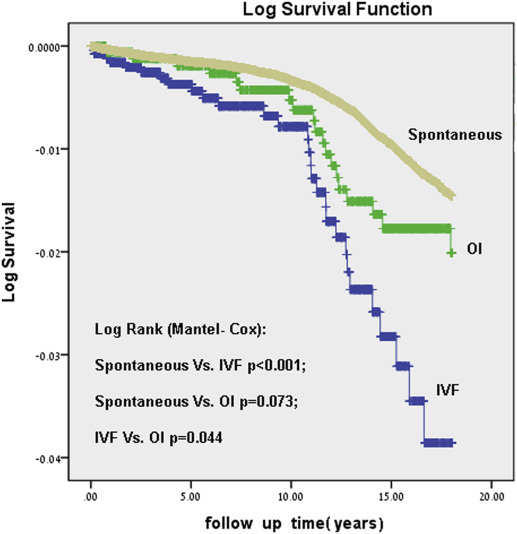
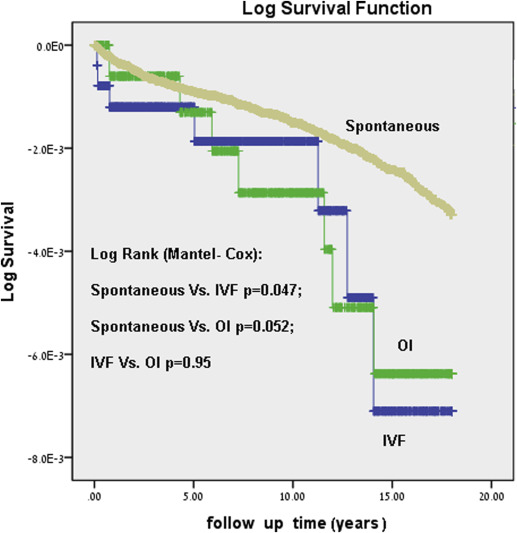
Table 3 presents results of the multivariable models by comparing the risk for hospitalization with neoplasms (malignancies and benign combined), malignancies, and benign tumors among the different study groups.
| Variables | Total neoplasm | Total benign | Total malignancies | |||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% Confidence interval | Hazard ratio | 95% Confidence interval | Hazard ratio | 95% Confidence interval | |
| Model 1 | ||||||
| Spontaneous | 1 | 1 | 1 | |||
| In vitro fertilization | 2.48 | 1.71–3.59 | 1.45 | 0.94–2.25 | 1.89 | 0.89–4.02 |
| Ovulation induction | 1.51 | 0.96–2.37 | 1.16 | 0.65–2.05 | 2.03 | 0.96–4.30 |
| Model 2: Any fertility treatments vs spontaneous | 1.97 | 1.47–2.63 | 1.33 | 0.94–1.89 | 1.96 | 1.14–3.36 |
| Model 3: In vitro fertilization vs ovulation induction | 0.58 | 0.31–1.07 | 0.74 | 0.33–1.68 | 0.96 | 0.31–2.93 |
| Model 4: In vitro fertilization vs else | 2.47 | 1.70–3.57 | 1.42 | 0.92–2.19 | 1.87 | 0.88–3.98 |
a Models were adjusted for maternal age, birthweight, preterm birth, pregnancy-related hypertensive disorders, and pregestational and gestational diabetes mellitus.
All model adjusted for maternal age, birthweight, diabetes mellitus (GDM or pre-GDM), hypertension (gestational or pregestational), and preterm births. The 2 later variables were included in the model, although they were not associated with the outcome, because of clinical significance.
The models show that, compared with spontaneously conceived children, the IVF group was at increased risk for total neoplasm hospitalization and that any fertility treatments were associated with a greater risk for total neoplasms and total malignancies. The IVF group was also at increased total neoplasm risk compared with the OI group and the spontaneously conceived children combined. The categoric time variable had no effect on the selected multivariable models and therefore was not included in the final models.
Results
During the study period, 242 187 newborn infants met the inclusion criteria: 2603 of whom (1.1%) were conceived after IVF; 1172 of whom (0.7%) were conceived after OI, and 237,863 of whom (98.3%) were with pregnancies that were conceived spontaneously. The rates of IVF have changed from 0.2% of the offspring in 1991 to 1.8–2.0% in the years 2012–2013; OI rates were 1.0% (1991) and 0.5% (2013). Table 1 presents background information of the study population, which includes demographic, obstetric, and perinatal characteristics. Mothers in the 3 study groups were different in terms of age, with IVF mothers being older than OI mothers, and both were older than the comparison group. In addition, both fertility treatment groups exhibited lower parity and gravidity, shorter mean gestation, and higher rates of preterm births and low birthweights. Pre-GDM and hypertension and GDM and hypertensive disorders were more prevalent among the fertility treatment groups. Mean follow-up time also differed by study group, with the longest follow-up time in the OI group.
| Characteristic | In vitro fertilization (n=2603; 1.1%) | Ovulation induction (n=1721; 0.7%) | Spontaneous (n=237,863; 98.3%) | P value |
|---|---|---|---|---|
| Maternal age at delivery, y a | 32.51±5.38 | 29.54±5.22 | 28.10±5.81 | <.001 |
| Gestational age, wk a | 38.25±1.89 | 38.74±1.96 | 39.16±1.74 | <.001 |
| Parity, n (%) | <.001 | |||
| 1 | 1355 (52.1) | 987 (57.4) | 54,790 (23.0) | |
| 2-4 | 1211 (46.5) | 697 (40.5) | 121,962 (51.3) | |
| ≥5 | 36 (1.4) | 37 (2.1) | 61,076 (25.7) | |
| Newborn gender (male), n (%) | 1288 (49.5) | 854 (49.6) | 120,973 (50.9) | .23 |
| Preterm delivery (gestational week <37), n (%) | 326 (12.5) | 161 (9.4) | 15,218 (6.4) | <.001 |
| Low birthweight (<2500 g), n (%) | 290 (11.1) | 196 (11.4) | 149,605 (6.3) | <.001 |
| Very low birthweight (<1500 g), n (%) | 25 (1.0) | 16 (0.9) | 759 (0.3) | <.001 |
| Small for gestational age, n (%) | 116 (4.5) | 118 (6.9) | 10,764 (4.5) | <.001 |
| Low Apgar 5 score (<7), n (%) | 21 (0.8) | 12 (0.7) | 4,274 (1.8) | <.001 |
| Mode of delivery, n (%) | <.001 | |||
| Cesarean | 1016 (39.0) | 445 (25.9) | 31,267 (13.1) | |
| Assisted | 118 (4.5) | 81 (4.7) | 7,590 (3.2) | |
| Vaginal | 1469 (56.4) | 1195 (69.4) | 199,006 (83.7) | |
| Pregestational diabetes mellitus, n (%) | 82 (3.2) | 53 (3.1) | 2,348 (1.0) | <.001 |
| Gestational diabetes mellitus, n (%) | 289 (11.1) | 171 (9.9) | 9,219 (3.9) | <.001 |
| Pregestational hypertension, n (%) | 89 (3.4) | 74 (4.3) | 3,105 (1.3) | <.001 |
| Gestational hypertensive disorders, n (%) | 265 (10.2) | 204 (11.9) | 11,638 (4.9) | <.001 |
| Follow-up time, d a | 2790.85±2028.9 | 4224.7±2147.0 | 3767.12±2157.43 | <.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


