Acute Kidney Injury in Infants
EPIDEMIOLOGY
Definition of Acute Kidney Injury
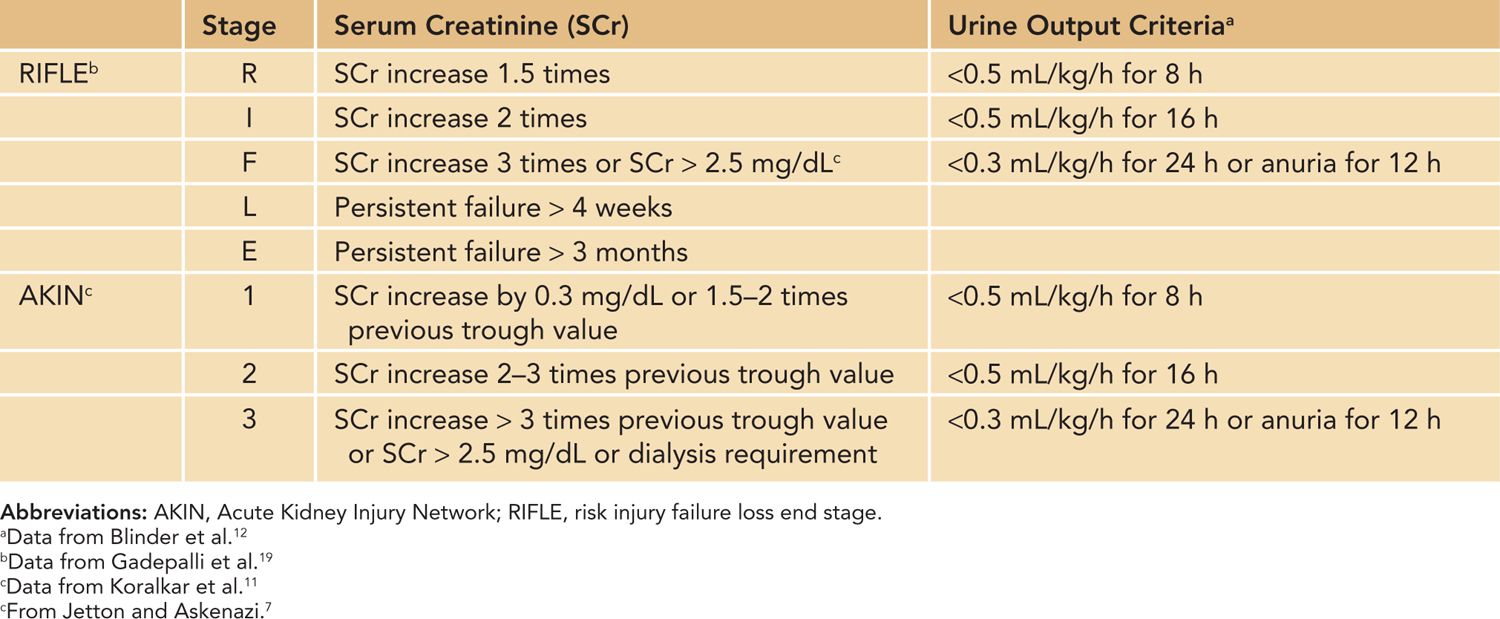
Acute kidney injury (AKI) is a condition defined by an acute decrease in the glomerular filtration rate (GFR), resulting in dysregulation of volume, electrolytes, acid-base balance and inability to excrete metabolic waste products. The term AKI has replaced the older term acute renal failure (ARF) to highlight the varying degrees of renal dysfunction, to enable clinicians to recognize renal dysfunction earlier in the patient’s course, and to allow researchers to apply a uniform definition of acute renal dysfunction.1 The Acute Dialysis Quality Initiative (ADQI) and Acute Kidney Injury Network (AKIN) groups standardized the definition of AKI in adult patients based on the increase in serum creatinine (Cr) or oligoanuria, and the pediatric community has adopted modified criteria based on the ADQI and AKIN definitions (Table 42-1).2
Table 42-1 Acute Kidney Injury Definitions Using RIFLE, pRIFLE, and AKIN
Definition of AKI in Neonates and Infants
There are additional important factors to consider in defining AKI in neonates using serum Cr and urine output criteria. First, neonatal serum Cr following birth reflects maternal Cr, and it decreases to a steady state over 1 to 2 weeks in term infants and over 3 to 4 weeks in preterm infants as the neonate’s GFR steadily improves.3,4 Tables 42-2 and 42-3 show the increasing GFR in preterm and term neonates.5,6 Second, unlike in critically ill adults or children, critically ill neonates may not have their serum Cr measured daily due to the concern for blood loss, and AKI may go unrecognized.7 Elevated bilirubin levels can interfere with the Jaffe reaction assay, a common laboratory method used to measure serum Cr, and lead to an underestimation in the serum Cr if the laboratory does not correct for the bilirubin interferences.8,9 Likewise, oliguria is not a sensitive marker for AKI in neonates because neonates commonly have nonoliguric AKI.7,10 In spite of these limitations, serum Cr and urine output measurements currently remain the main clinical tools in identifying and diagnosing AKI in neonates. Jetton et al have proposed a standardized definition of neonatal AKI based on the AKIN criteria,7 and recent studies in neonatal AKI have utilized modified RIFLE (risk injury failure loss end stage) criteria, pediatric RIFLE (pRIFLE), and the AKIN criteria to define AKI.11–13 Table 42-4 shows the modified definitions used in these studies. The following modifications were made: (1) The upper cutoff Cr of 4.0 mg/dL in stage 3 (AKIN) and stage F failure (RIFLE) is lowered to 2.5 mg/dL to reflect the neonates’ lower muscle mass and lower steady-state baseline Cr.11 (2) The 6-hour period used in the urine output criteria is changed to an 8-hour period to be consistent with the pRIFLE definition of oligoanuria, which was validated in pediatric patients.12 These proposed definitions of neonatal AKI are promising alternatives to the current practice of arbitrarily defining AKI, but these definitions need to be validated to determine their clinical utility.
Table 42-2 Glomerular Filtration Rate (GFR) by Inulin Clearance in Preterm and Term Infantsa
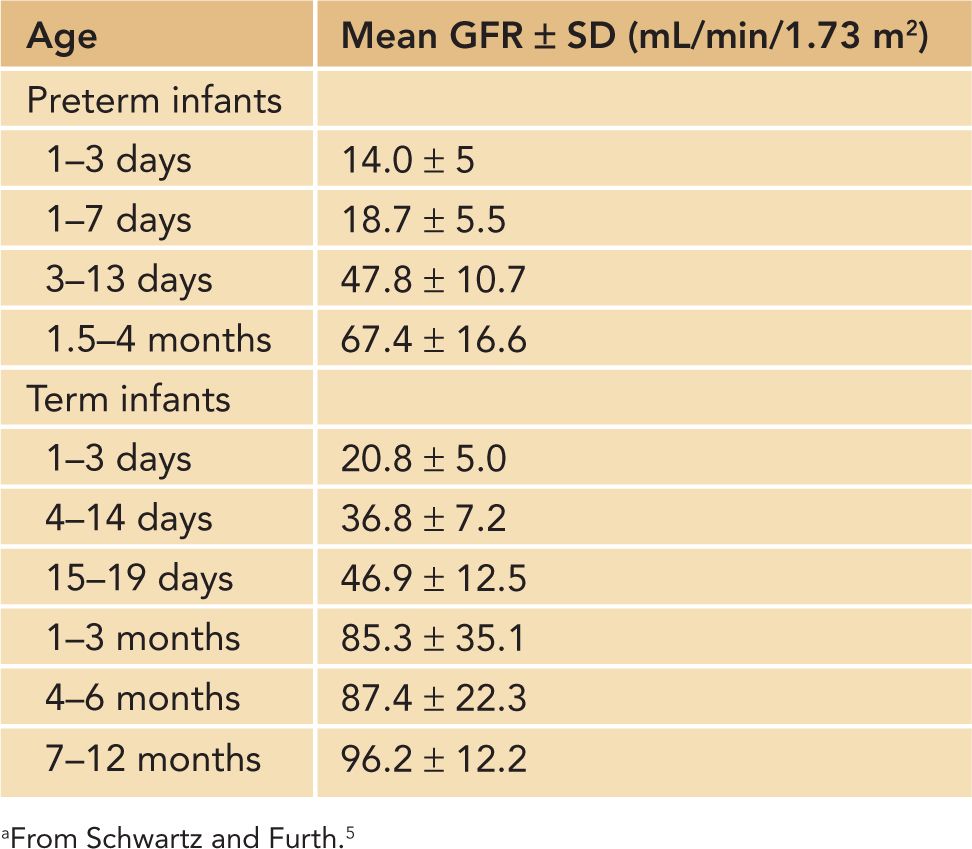
Table 42-3 Glomerular Filtration Rate (GFR) by Creatinine Clearance in Very Preterm Infants During First Month of Life
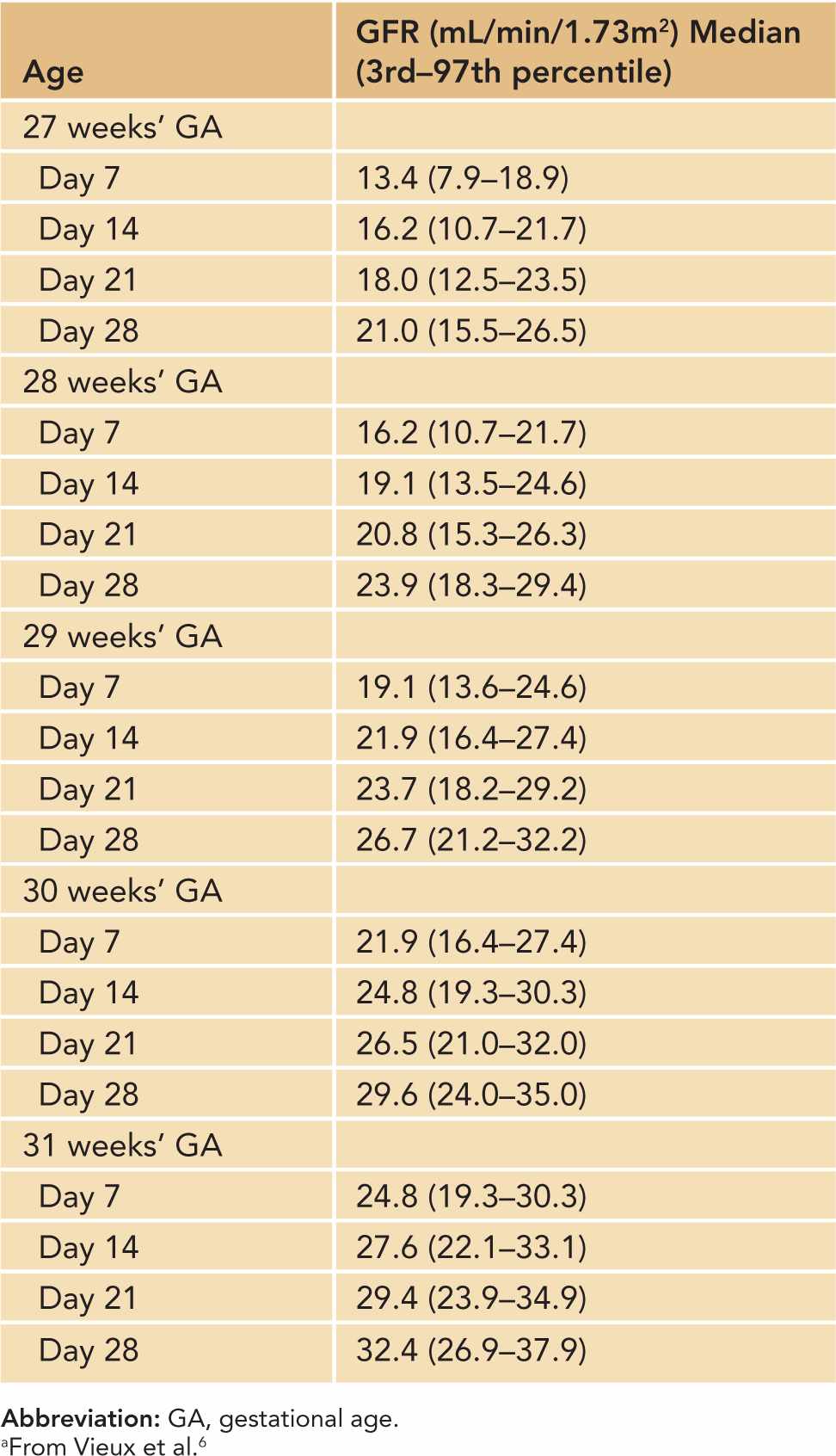
Table 42-4 Proposed Definitions and Classification of AKI in Neonates and Infants
Incidence of AKI in Neonates and Infants
Acute kidney injury has been reported in 3.4%–24% of the neonates admitted to neonatal intensive care units (NICUs).10,11,13 The true incidence of neonatal AKI is unknown, however, and it is believed to be higher than the reported values since most studies of neonatal AKI use a high serum Cr value or the need for dialysis to define AKI, thus failing to include those with mild and moderate forms of AKI.14,15
Prematurity
In a prospective study of very low birth weight (VLBW) neonates (birth weight 500–1500 g), AKI was found in 18% of the cohort using modified AKIN AKI criteria.11 AKI was reported in 12.5% of extremely low birth weight (ELBW) neonates in a retrospective case-control study, but mild, moderate, and nonoliguric AKI were unidentified in this study because the study defined AKI as serum Cr greater than 1.5 mg/dL or oliguria of less than 1 mL/kg/h.13
Perinatal Asphyxia
Acute kidney injury is reported in 36.1%–56% of neonates with perinatal asphyxia, variously defined15–17 as 1-minute Apgar score below 7 or 5-minute Apgar scores less than 6 to 7. The incidence of AKI increases with the severity of asphyxia: AKI was reported in 9.1% of infants with moderate asphyxia (1-minute Apgar score 4–6) and in 56% of infants with severe asphyxia (1-minute Apgar score < 3).15
Extracorporeal Membrane Oxygenation
In a large retrospective study using data from the Extracorporeal Life Support Organization (ELSO) Registry, AKI was found in 8% (638/7941) of infants who received extracorporeal membrane oxygenation (ECMO) for noncardiac reasons; AKI was defined as serum Cr greater than 1.5 mg/dL, diagnostic code for ARF, or procedural code for dialysis.18 AKI, defined by modified RIFLE, was reported at a much higher rate of 71% (48/68) in a single-center retrospective study of neonates who received ECMO for congenital diaphragmatic hernia (CDH).19 The large disparity in the AKI incidence in the 2 studies is likely due to the varying definitions of AKI.
Cardiac Surgery for Congenital Heart Disease
Two recent large studies showed that postoperative AKI, defined by the modified AKIN criteria, occurs commonly (52%–62%) in infants undergoing cardiac repair surgery for congenital heart disease.12,20 The following factors were associated with postoperative AKI in multivariate analyses: single-ventricle physiology (odds ratio [OR] 1.6; 95% confidence interval [CI] 1.1, 2.4); the need for cardiopulmonary bypass (CPB) (OR 1.2; 95% CI 1.01, 1.5)12; CPB duration longer than 180 minutes (OR 5.63; 95% CI 1.13, 28); deep hypothermic circulatory arrest (DHCA) (OR 2.14; 95% CI 1.04, 4.43); and the presence of chromosome abnormality (OR 4.4; 95% CI 1.26, 15.4).20
PATHOPHYSIOLOGY
The pathophysiology of AKI is complex, and it involves an intricate interplay of various mechanisms involving exogenous and endogenous toxins, hemodynamic instability in renal macro- and microcirculation, hypoxia, ischemia-reperfusion injury, inflammation, and oxidative stress. A complete review of the various pathophysiologic mechanisms of AKI is beyond the scope of this chapter. A few key mechanisms pertinent to neonatal AKI are highlighted in this section.
Incomplete Nephrogenesis in Prematurity
Nephrogenesis begins at 4–5 gestational weeks and completes at 34–36 gestational weeks.4,21 Importantly, autopsy studies have shown that nephrogenesis and glomerulogenesis do not continue normally in the extrauterine environment for prematurely born infants. Prematurely born infants (27 ± 3 gestational weeks) were autopsied at 63 ± 27 weeks postconceptual age. Their glomerulogenesis, as measured by radial glomerular counts (RGCs), was found to be significantly lower than in term infants autopsied at 40 ± 1 weeks postconceptual age, suggesting that extrauterine glomerulogenesis in prematurely born infants is suboptimal when compared to intrauterine glomerulogenesis seen in term infants.22 Moreover, glomerulogenesis in premature infants with AKI was noted to be significantly less than in premature infants without AKI.22
Impaired Autoregulatory Mechanisms Increase the Risk of Prerenal Injury
Renal blood flow (RBF) is determined by cardiac output (CO) and renal perfusion pressure. Renal perfusion pressure is approximately equal to systemic arterial blood pressure (BP) and renal vascular resistance (RVR), which is largely determined by the afferent and efferent arterioles.23 RBF can be expressed as RBF = BP/RVR.23
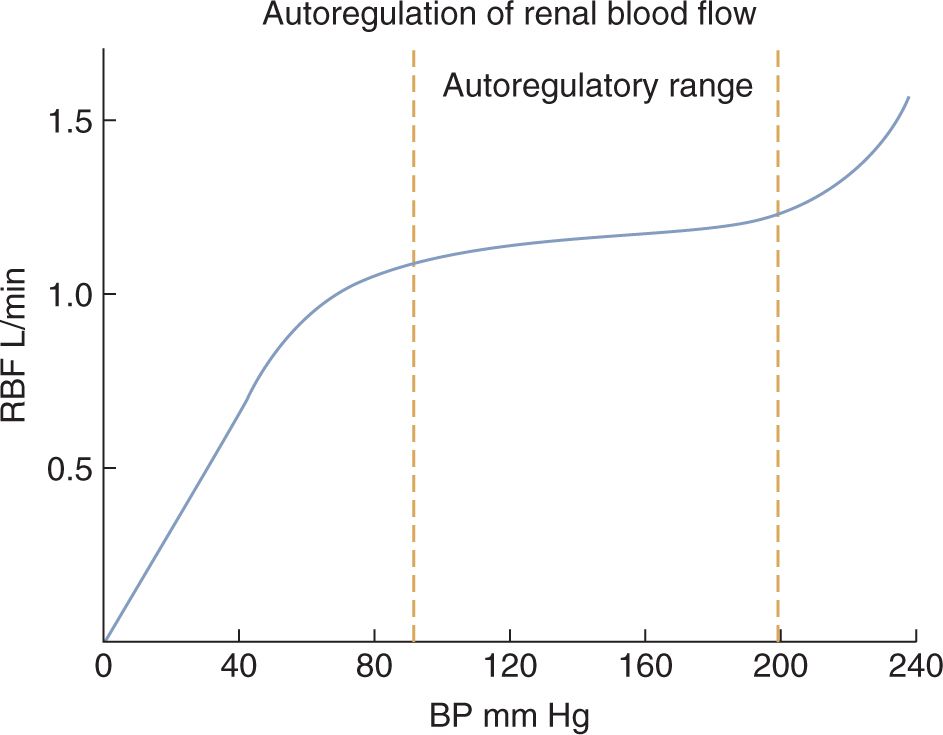
Renal blood flow and GFR change only modestly when the renal perfusion pressure (ie, BP) changes within the range that is commonly found in humans (Figure 42-1).24 This tight regulatory control of RBF and GFR are due to autoregulatory mechanisms from the myogenic response and tubuloglomerular feedback, and these mechanisms maintain RBF and GFR in the setting of decreased renal perfusion.23–25 When renal perfusion decreases, the afferent arteriole vasodilates, which lowers the RVR and maintains RBF. Efferent arteriolar vasoconstriction maintains the glomerular pressure (and therefore filtration) in the face of reduced total glomerular blood flow. Decrease in renal perfusion stimulates the generation of prostaglandins (causing afferent vasodilation), catecholamine secretion, and renin-angiotensin system activation (both causing efferent constriction), which all contribute to maintenance of GFR.
FIGURE 42-1 Autoregulation allows the renal blood flow (RBF) to be maintained at a relatively steady rate during changes in blood pressure within the common range. Glomerular filtration rate follows a similar pattern as the RBF. (Reproduced with permission from Kibble J, Halsey CR. Medical Physiology: The Big Picture. New York: McGraw-Hill; 2009.)
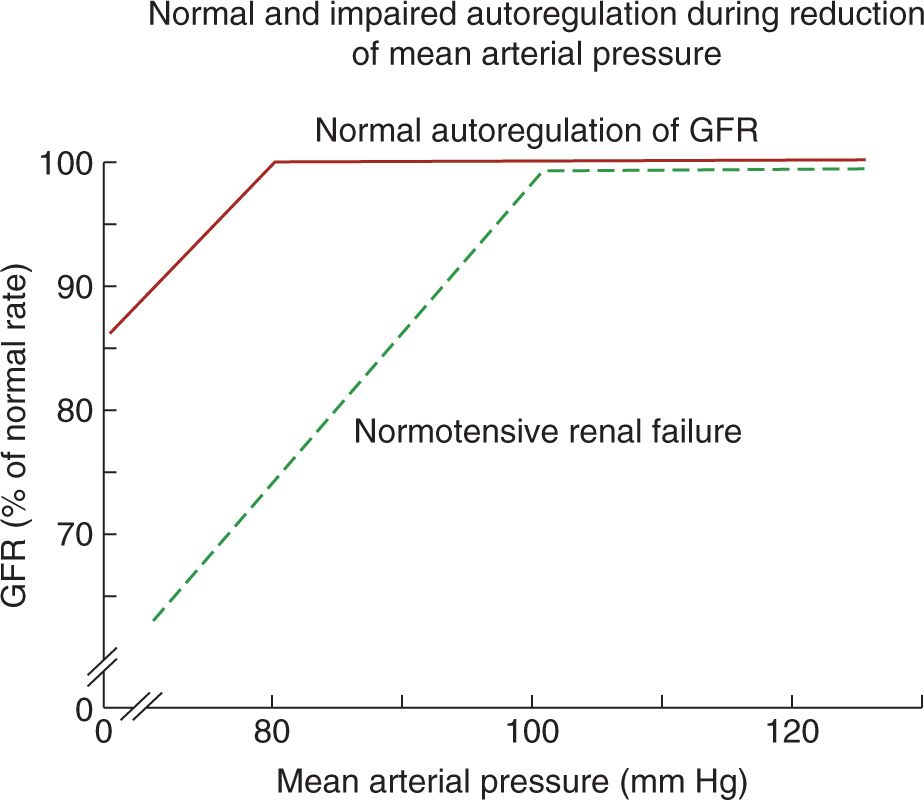
In adults with intact autoregulation, GFR is maintained until the mean arterial pressure decreases below 80 mm Hg. In those with impaired autoregulation, however, GFR decreases even in the setting of normal BPs, resulting in normotensive AKI (Figure 42-2).26 Therefore, in patients with impaired autoregulation, higher renal perfusion pressure (ie, systemic BP) is needed to maintain RBF and GFR. Table 42-5 lists conditions that may impair renal autoregulation and result in increased susceptibility to AKI even in settings of moderate hypoperfusion.26 Neonates on indomethacin and furosemide therapies for persistent ductus arteriosus (PDA), for example, have impaired renal autoregulation due to indomethacin’s inhibition of prostaglandin production as well as furosemide’s inhibition of Na+/K+/2Cl− cotransporter, which is involved in the tubuloglomerular feedback mechanism.25,27
FIGURE 42-2 In normal autoregulation, glomerular filtration rate (GFR) is maintained until the mean arterial pressure decreases below 80 mm Hg. In patients with impaired autoregulation, however, GFR decreases even when the mean arterial pressure is within normal limits. (From Abuelo.26)
Table 42-5 Factors That Impair Renal Autoregulation and Increase Susceptibility to Acute Kidney Injury
Inability to decrease arteriolar resistance
Reduction in vasodilatory prostaglandins
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Cyclooxygenase 2 inhibitors
Afferent glomerular arteriolar vasoconstriction
Sepsis
Hypercalcemia
Hepatorenal syndrome
Cyclosporin or tacrolimus
Radiocontrast agents
Failure to increase efferent arteriolar resistance
Angiotensin-converting enzyme inhibitors (ACEIs)
Angiotensin receptor blockers (ARBs)
Decreases tubuloglomerular feedback
Furosemide
ACEIs and ARBs
Renal artery stenosis
a From Abuelo.26
Inflammation and Oxidative Stress in AKI
Experimental AKI models in ischemia-reperfusion, sepsis-endotoxemia, and nephrotoxin injury have shed further light in the pathogenesis of AKI. The initial insult from ischemia, sepsis, and nephrotoxin results in morphologic and functional changes to endothelial cells and tubular epithelial cells, which in turn initiate the proinflammatory cascade.28 In the endothelium, for example, ischemic injury disrupts the cell-to-cell border, leading to impaired barrier function and increased vascular permeability, which facilitates leukocyte infiltration into the injured parenchyma.28 Injured proximal tubular epithelial cells express interferon regulatory factor 1 (IRF-1), a transcription factor that activates proinflammatory genes, including interferons and chemokines.29 Injured cells and leukocytes produce proinflammatory cytokines/chemokines, such as tumor necrosis factor α (TNF-α), interleukin (IL) 2, IL-6, IL-10, and monocyte chemoattractant protein-1 (MCP-1), which are important in the initiation and extension of inflammation in AKI.28
The combination of reactive oxygen species (ROS) and impaired antioxidant capacity are believed to contribute significantly to the pathogenesis in AKI. Under normal circumstances, ROS such as hydroxyl radical (HO−), peroxynitrite (ONOO−), and hyperchlorous acid (OCl−) are generated in low concentrations and are neutralized by endogenous antioxidants, including catalase and glutathione. In hypoxemic and inflammatory settings, however, the homeostasis between the oxidants and antioxidants is altered to favor oxidants, leading to tissue injury.25,30 These ROSs attack various cellular components and cause disruptions in the plasma membrane, cytoskeletal proteins, and cell-to-cell adhesions.25,31 ROSs also scavenge nitric oxide and cause vasoconstriction.25
DIFFERENTIAL DIAGNOSES
The causes of AKI are classically divided into 3 categories: prerenal, intrinsic, and postrenal (obstructive) (Table 42-6). Prerenal azotemia is considered a functional response to renal hypoperfusion, and renal microstructure and parenchyma are preserved. Prerenal azotemia resolves within a few days of restoring adequate renal perfusion. Intrinsic AKI can be further subdivided into vascular (large vessels and microvascular), glomerular, and tubulointerstitial causes. Despite this classification system, it is important to recognize that AKI in the clinical setting is often multifactorial, and that the risk of AKI increases with the number of nephrotoxic insults.27,32 A few specific causes of AKI commonly seen in neonates are discussed.
Table 42-6 Causes of Acute Kidney Injury in Neonates and Infants
Prerenal
Intravascular volume depletion
• Gastrointestinal losses: vomiting, diarrhea, nasogastric tube loss, ostomy loss
• Renal losses: diuretic use, osmotic diuresis, diabetes insipidus
• Hemorrhage: twin-twin transfusion, surgery
• Skin, mucous membrane losses
• Third-space loss: capillary leakage, sepsis
Decreased renal blood flow/perfusion
• Hypotension
• Cardiogenic shock
• ECMO
• Cardiopulmonary bypass surgery
• Congenital heart disease: persistent ductal arteriosus (large left-to-right shunt)
• Perinatal asphyxia
• Abdominal compartment syndrome
Renal vasoconstriction
• Hepatorenal syndrome
• Acute hypercalcemia
• Drugs: NSAIDs, norepinephrine, vasopressin
• Iodinated contrast agents
Intrinsic
Acute tubular necrosis/tubular injury
• Prolonged ischemia
• Endogenous toxins: myoglobin, hemoglobin, uric acid
• Exogenous toxins: antibiotics, radiocontrast agents, phosphate preparations
Acute tubulointerstitial nephritis
• Drugs: NSAIDs, antibiotics
• Infections: bacterial, viral, fungal infections
Vascular lesions
• Cortical necrosis
• Renal vein thrombosis
• Renal artery thrombosis
Congenital/Genetic
Hypoplasia
• Dysplasia with or without obstructive uropathy
• Cystic renal diseases
– ARPKD
– ADPKD (rare to have renal dysfunction in infancy)
– Cystic dysplasia
• Congenital nephrotic syndrome
Postrenal/Obstructive
• Obstructive uropathy: posterior urethral valves, neurogenic bladder
• Obstruction of ureters, bladder, or urethra
• Retroperitoneal mass
• “Pseudo-AKI”: urinary tract perforation
Abbreviations: AKI, acute kidney injury; ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; ECMO, extracorporeal membrane oxygenation; NSAID, nonsteroidal anti-inflammatory drug.
Aminoglycoside-Induced AKI
Kidneys are susceptible to drug-induced toxicity due to their high blood flow, their role in drug metabolism and elimination, and high concentrations of the drug in the tubular lumen and interstitium.25 In aminoglycoside nephrotoxicity, the cationic amino groups on the drug bind to the anionic phospholipid residue on the proximal tubule. The drug is then internalized through endocytosis and sequestered in the lysosomes, where it causes lysosomal rupture and leads to mitochondrial dysfunction, impaired protein synthesis, and cell apoptosis.33–35 Aminoglycoside-induced nephrotoxicity typically causes nonoliguric AKI with tubular dysfunction, which can lead to multiple electrolyte anomalies, such as hypokalemia, hypophosphatemia, and hypomagnesemia.35 The risk factors for developing aminoglycoside-induced AKI in neonates include prolonged duration of therapy (>5–10 days), high trough and peak levels, concomitant nephrotoxic medications, and the presence of renal hypoperfusion.35
Nonsteroidal Anti-inflammatory Drug-Induced AKI
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as indomethacin and ibuprofen, decrease RBF by inhibiting cyclooxygenase (COX) enzyme, thus inhibiting the production of prostaglandins, vasodilators in renal circulation. NSAIDs can also cause kidney injury independent of the cyclooxygenase inhibition. In vitro studies have shown that indomethacin causes renal epithelial cell injury by downregulating its antiapoptotic proteins and upregulating proapoptotic proteins.36 Likewise, it is well known that NSAIDs can cause interstitial nephritis in humans.37 In a Japanese postmarking surveillance study consisting of 2538 low birth weight infants, electrolyte and renal abnormalities (defined as Na < 125 mEq/L, K > 7 mEq/L, urine output decrease > 40%, serum urea nitrogen [BUN] > 40 mg/dL, or Cr > 1.7 mg/dL) were seen in 40% of the infants following indomethacin administration.38 Risk factors associated with the development of electrolyte abnormality or renal insufficiency included maternal tocolysis with indomethacin (OR 1.4; 95% CI 1.01, 1.95); chorioamnionitis (OR 1.39; 95% CI 1.04, 1.86); and preexisting electrolyte or renal abnormalities (OR 1.23; 95% CI 1.23, 2.73).38
Some have hypothesized that furosemide may attenuate the nephrotoxic side effects of indomethacin as furosemide was found to stimulate COX-2, resulting in increased prostaglandin E2 (PGE2) production.39–41 However, recent clinical studies have shown a higher incidence of AKI in neonates who received indomethacin with furosemide compared to those who received indomethacin alone for PDA.42,43 In a single center, a randomized, prospective study of indomethacin plus furosemide vs indomethacin alone in preterm infants with PDA, AKI (defined as serum Cr > 1.6 mg/dL) was seen in 59% of those who received indomethacin and furosemide and in 10% of those who received indomethacin alone (OR 12.38; 95% CI 3.1, 49.0).43 In fact, the study was terminated early due to the significantly higher risk of AKI in the indomethacin-plus-furosemide group. In these neonates, who often have multiple risk factors for AKI, such as prematurity, respiratory distress syndrome, exposure to nephrotoxic medications, and renal hypoperfusion due to large shunting via PDA, furosemide may act as an additive nephrotoxic insult by causing hypovolemia and further impairment in renal autoregulation.
Ibuprofen has fewer nephrotoxic side effects than indomethacin and a closure rate similar to PDA.44 In a prospective, multicenter, randomized trial of indomethacin vs ibuprofen in 148 infants with PDA, oliguria (urine output < 1 mL/kg/h) occurred in 19% of the infants in the indomethacin group and in 7% of the infants in the ibuprofen group (P = .03). Serum Cr was 1.09 mg/dL in the indomethacin group and 0.95 mg/dL in the ibuprofen group (P = .04) 4 days following therapy.45 A Cochrane database review in 2010 examined the efficacy and adverse effects of indomethacin vs ibuprofen in PDA closure in preterm infants and concluded that the PDA closure failure rate was similar between the 2 agents and that transient renal insufficiency was seen less in the ibuprofen group.46 Although the mechanisms that differentiate the nephrotoxic effects of indomethacin and ibuprofen are unclear, 1 hypothesis is that indomethacin may inhibit COX-1, which is believed to have a more prominent role in the renal basal physiologic processes than COX-2, more than ibuprofen.44,45
DIAGNOSTIC TESTS
History and Physical Examination
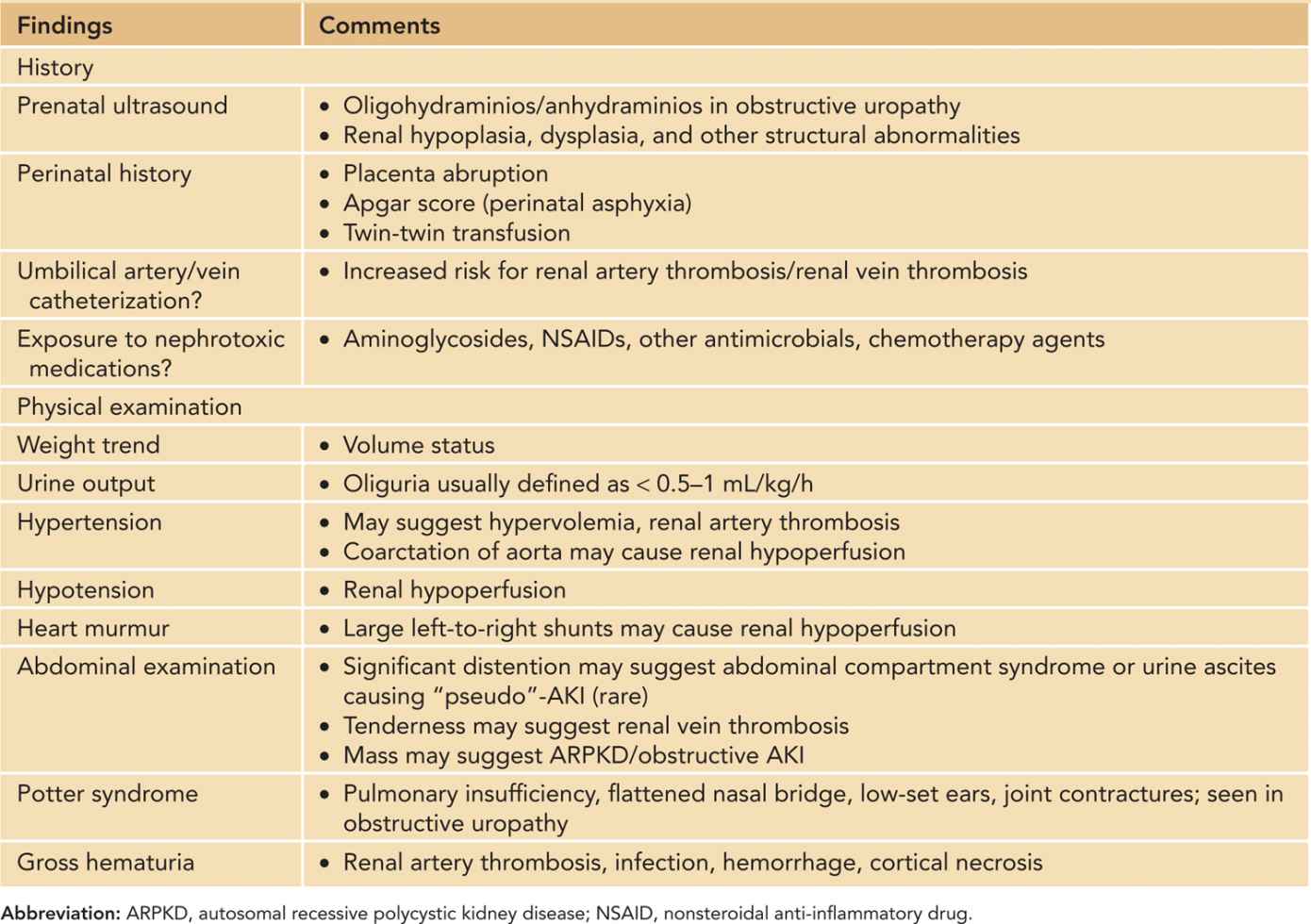
Prenatal history, perinatal history, and physical examination are important in the workup of a neonate with AKI. Table 42-7 summarizes pertinent history and physical examination findings. A urethral Foley catheter not only can facilitate in the strict in-and-out fluid measurements but also can be used to diagnose obstructive AKI. In addition, intravesical pressure can be measured in clinically suspected cases of abdominal compartment syndrome. The World Society of Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as sustained intra-abdominal pressure greater than 20 mm Hg associated with new organ dysfunction or failure.47 In children and infants, however, abdominal compartment syndrome has been described in patients with lower intra-abdominal pressures (10–12 mm Hg).48,49
Table 42-7 Pertinent History and Physical Examination in Neonates/Infants With Acute Kidney Injury
Laboratory Evaluation
Serum Creatinine
Serum Cr is a marker of renal dysfunction and late consequence of injury, not a marker for injury itself. Serum Cr may not change until 25%–50% of the kidney function has been lost, thus relying only on serum Cr delays the diagnosis of true kidney injury. Also, at lower GFR levels, serum Cr overestimates the true GFR as tubular secretion of Cr increases.14 Nonetheless, serum Cr is the most commonly used biomarker to identify AKI in the clinical setting.
Urine Studies
Fractional excretion of sodium (FeNa) is less than 2.5% in prerenal azotemia and more than 2.5% in intrinsic AKI in neonates.50,51 FeNa is calculated as [(UNa × PNa)/(UCr × PCr)] × 100. Fractional excretion of urea (FeUrea) less than 35% has been associated with prerenal azotemia in adult patients; however, this has not been studied in neonates.52 Similar to FeNa, FeUrea is calculated as [(Uurea × PBUN)/(UCr × PCr)] × 100.
Plasma and Urine Biomarkers
Various biomarkers, including cystatin C (CysC), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), and IL-18 have been studied in neonates in attempts to identify kidney injury early and allow for earlier intervention with the ultimate goal of improving morbidity and mortality. These biomarkers have been studied in the clinical settings of asphyxia, cardiac surgery, sepsis, and nephrotoxic exposures. Although the majority of the results are promising for the biomarkers’ ability to predict AKI earlier than serum Cr in the neonatal population, biomarkers continue to remain primarily a research interest and have not yet been widely incorporated into daily clinical use.
Cystatin C. CysC is an endogenous cysteine protease inhibitor produced by most nucleated cells at a constant rate. It is freely filtered by the glomerulus, not secreted by the renal tubules, and completely reabsorbed and catabolized in the proximal tubule.53,54 Unlike serum Cr, serum CysC levels in term infants do not reflect maternal serum values, making CysC an attractive alternative for estimating GFR in neonates.54 Although serum CysC has been shown to better estimate GFR in children, this remains to be seen in neonates.55–57
Urine CysC concentration has been found to be elevated in neonates with AKI. In a nested case-control study of neonates with 5-minute Apgar scores less than 7, urine CysC concentration in 9 neonates with AKI was 1771% higher than in 24 neonates without AKI.58 The area under the curve (AUC) was 0.82, indicating good positive prediction for AKI. Li et al prospectively collected urine biomarkers from nonseptic critically ill neonates and showed that urine CysC levels collected 0–48 hours prior to the clinical diagnosis of AKI was significantly higher in 11 neonates with AKI compared to 51 neonates without AKI.59 For every 1000 ng/mg increase in urine CysC/urine Cr, the adjusted OR of developing AKI within 48 hours was 2.18 (95% CI 1.16, 4.12), with an excellent AUC of 0.92.
Neutrophil gelatinase-associated lipocalin. NGAL is a protein expressed in renal tubules after ischemic or toxic injury.54 There is a rapid upregulation of NGAL messenger RNA (mRNA) in the proximal tubules and in the loop of Henle within a few hours of nephrotoxic stimuli.60 Among 35 neonates (>37 gestational weeks) undergoing cardiac surgery, plasma and urine NGAL concentrations were elevated 2 hours following CPB initiation in 8 neonates who developed AKI compared to 27 neonates who did not develop AKI.61 For every 10 ng/mL increase in the 2-hour post-CPB initiation plasma NGAL level, the odds of AKI increased by 47% (OR 1.47; 95% CI 1.29, 1.73). Likewise, for every 10 ng/mL increase in the 2-hour urine NGAL concentration, the odds of AKI increased by 32% (OR 1.32; 95% CI 1.17, 1.52). In a small case-control study of neonates with perinatal asphyxia with and without AKI, plasma and urine NGAL concentrations were noted to be significantly elevated in those with AKI when checked on day of life (DOL) 1, 3, and 10.62 Urine NGAL has also been found to be elevated in VLBW infants with and without AKI.63 For every 100 ng/mL increase in NGAL, the odds of having AKI increased by 20% (OR 1.2; 95% CI 1.0, 1.6; P <.01). It is important to note that the absolute NGAL cutoff values in the 3 studies mentioned differed notably from 18.6 ng/mL62 to 985 ng/mL63 using the enzyme-linked immunosorbent assay (ELISA) method. This may be due to the age of the infants at the time of the sampling, their gestational age, interassay variations between different commercial assays, and possibly the different underlying pathologic processes that led to the common clinical outcome of AKI as defined by serum Cr.
Kidney injury molecule 1. KIM-1 is a transmembrane glycoprotein expressed on renal tubules following ischemic or toxic injury. It confers on the epithelial cells the ability to recognize and phagocytose dead cells.64 Urine KIM-1 levels were not significantly different in asphyxiated neonates with and without AKI on DOL 1 or 3, but the levels were higher on DOL 10.62 Urine KIM-1 levels were not significantly different in VLBW infants with and without AKI, but urine KIM-1 level was higher in nonsurvivors than in survivors.63
Imaging Studies
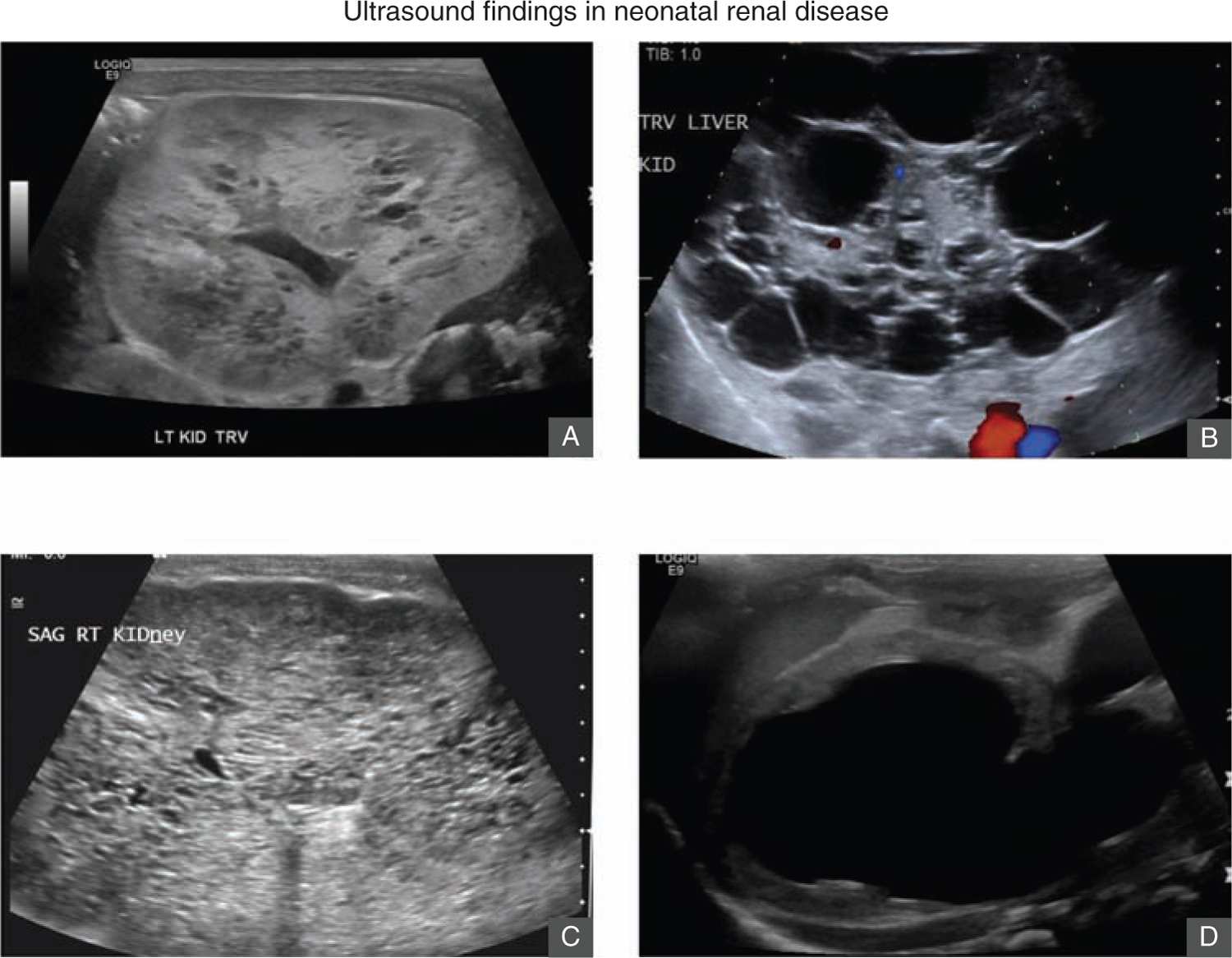
Renal ultrasound is an important initial imaging study in evaluating neonates with AKI. It can identify congenital renal disease such as ARPKD or obstructive causes of AKI (Figure 42-3). Pertinent features of the parenchyma include kidney size and volume, echogenicity, corticomedullary differentiation, and cortical thickness.65 The presence of severe bilateral urinary tract dilation and hydronephrosis may suggest obstructive causes of AKI. Patients with acute tubular necrosis or other causes of intrinsic AKI may have increased echogenicity of the cortex, but more commonly, the ultrasound is normal. Doppler study should also be obtained to evaluate the blood flow to the kidneys. Noncontrast MRI may be needed to more accurately characterize renal perfusion if there are strong clinical concerns for renal infarcts, renal vein thrombosis, or renal artery stenosis. Echocardiogram is important in neonates suspected to have congenital heart disease or heart failure as AKI can occur in the setting of cardiorenal syndrome.66,67
FIGURE 42-3 Ultrasound findings in renal disease. A, Renal ultrasound of a 2-day-old term girl with increasing serum creatinine and anuria. Renal ultrasound shows bilateral cystic dysplasia. She was started on peritoneal dialysis. B, Renal ultrasound of a day-old boy born at 29 gestational weeks with increasing serum creatinine and anuria. Renal ultrasound shows bilateral multicystic kidneys. He underwent bilateral nephrectomy and was started on peritoneal dialysis. C, Renal ultrasound of a day-old term boy with elevated serum creatinine and anuria. Renal ultrasound shows large echogenic kidneys with innumerable small cysts consistent with autosomal recessive polycystic kidney disease. D, Renal ultrasound of a few-day-old boy born at 36 gestational weeks with pulmonary hypoplasia and Potter syndrome. He was found to have posterior urethral valves. This ultrasound shows a large urinoma in the left kidney with little parenchymal tissue.
MANAGEMENT
The management of AKI, in most cases, depends on the etiology of AKI. In prerenal AKI, fluid resuscitation is required to restore renal perfusion and renal function. In obstructive AKI, the creation of a lower-resistance urinary flow tract by placing a urinary Foley catheter or pylostomy may be required. As the risk and the severity of AKI increase with the number of nephrotoxic insults, it is important to minimize these insults by removing nephrotoxic medications and preventing hypovolemia and hypotension to maintain renal perfusion. As discussed, many infants with AKI have decreased ability to autoregulate RBF; therefore, they often require higher BPs to maintain adequate renal perfusion.
Acute Management of Intrinsic AKI
There is no specific treatment of AKI; therefore, the management focus is interventions to prevent the development of AKI and to minimize the degree of renal injury once AKI occurs. A few interventions have shown promising results in neonates with AKI.
Rasburicase
Recent data from animal model, clinical, and epidemiological studies have suggested a pathogenic role of uric acid in the development and progression of kidney disease.68,69 Uric acid may cause vasoconstriction, impair autoregulation, decrease GFR, and stimulate proinflammatory responses.70 Rasburicase is a recombinant urate oxidase enzyme that converts existing uric acid to allantoin, which is highly water soluble and readily excretable.71 In a small retrospective study consisting of 7 neonates with AKI and hyperuricemia (serum uric acid > 8 mg/dL), a single intravenous dose of rasburicase (0.15–0.20 mg/kg) followed by normal saline flush was administered. The cause of AKI in these neonates included hypoxic ischemic event, sepsis with and without multiorgan failure, and acute tubular necrosis (ATN). Serum uric acid decreased significantly within 24 hours, mean serum Cr decreased from 3.2 ± 2 mg/dL to 2.0 ± 1.2 mg/dL (P < .05) within 24 hours, and urine output increased from 2.4 ± 1.2 mL/kg/h to 5.9 ± 1.8 mL/kg/h (P < 0.05).72 This study, however, lacked a control group. Although there are other case reports of hyperuricemic AKI improvement following rasburicase administration in pediatric patients, a prospective randomized study is needed.73–75 Rasburicase should be avoided in patients with glucose-6-phosphate dehydrogenase deficiency, methemoglobinemia, and other conditions known to cause hemolytic anemia.71
Theophylline
Renal production of adenosine increases following ischemic injury, and renal adenosine causes afferent arteriolar vasoconstriction and efferent arteriolar vasodilation, leading to a decrease in GFR.76 Theophylline and aminophylline are methylxanthine derivatives that act as nonspecific adenosine receptor antagonists and have been shown to decrease renal injury in animal models77 and decrease contrast-induced nephropathy in patients.78 A single dose of theophylline was shown to decrease the incidence of AKI in term infants with perinatal asphyxia in a randomized, double-blind, and placebo-controlled study.79 Twenty-four infants were randomized to receive theophylline (8 mg/kg IV), and 27 infants were randomized to receive placebo within an hour of delivery. AKI developed in 4/24 (17%) infants in the theophylline group compared to 15/27 (55%) infants in the placebo group (relative risk [RR] 0.30; 95% CI 0.12, 0.78; P < .001). Serum Cr was similar between the 2 groups on day of life (DOL) 1, but it was higher in the placebo group during the remaining study period from DOL 2 to 5. Urine output was significantly higher in the theophylline group during the first 4 DOL than in the placebo group. Serum theophylline level was 12.7 μg/mL (range 7.5–18.9 μg/mL) in the treatment group at 36–48 hours compared to 0.87 μg/mL in the placebo group. In a similar randomized, placebo-controlled study of 70 term infants with perinatal asphyxia, 40 infants received a single intravenous dose of theophylline (8 mg/kg), and 30 infants received a single dose of placebo within 1 hour of birth. AKI was seen in 10/40 (25%) of infants in the treatment group compared to 18/30 (60%) of infants in the placebo group (RR 0.41; 95% CI 0.22, 0.76; P < .001).80 Urine output was significantly higher in the treatment group on DOL 2 and 3, and the fluid output/input ratio was greater in the treatment group during the first 5 DOL. Serum Cr normalized by 6 weeks of life in all infants with AKI. At 1-year follow-up, serum Cr and Cr clearance were similar in both groups. In a randomized, double-blind, placebo-controlled study of 47 preterm (<32 gestational weeks) infants with respiratory distress syndrome requiring mechanical ventilation or nasal continuous positive airway pressure, the infants who received theophylline (1 mg/kg IV daily for 3 days) had higher urine output on DOL 1 than those in the placebo group, but there was no difference in the urine output between the 2 groups over the remaining 10 days of the study period.81 Serum Cr was significantly lower in the theophylline group on DOL 2, but serum Cr levels were similar on DOL 5 and DOL 11. Current evidence shows that theophylline improves urine output and estimated GFR in the early course of the AKI. Long-term effects on clinically important outcomes such as mortality and hospital length of stay were not examined in these studies. At our center, we administer a single loading dose of 5 mg/kg IV of aminophylline followed by 1.8 mg/kg IV every 6 hours to target a serum trough theophylline level of 5–7 μg/mL. A study of the effectiveness of aminophylline treatment in prevention of AKI in high-risk newborns requiring CPB is ongoing in our center (Clinicaltrials.gov, NCT01245595).
Fenoldopam
Fenoldopam is a selective dopamine 1 receptor agonist that has been shown to induce renal vasodilation, diuresis, and natriuresis.82 Randomized studies in critically ill adult patients have shown mixed results, with some showing reduction in the incidence of AKI83,84 and some showing no benefit.85,86 Recent meta-analyses showed that fenoldopam reduced AKI in adult patients undergoing cardiac surgery87 and reduced AKI, the need for renal replacement therapy (RRT), and in-hospital mortality in critically ill adult patients.88 A retrospective study of 13 critically ill pediatric patients showed that fenoldopam increased diuresis without adverse effects such as hypotension.89 In a prospective, nonblinded clinical trial consisting of 40 neonates undergoing cardiac surgery requiring CPB, 20 neonates were assigned to receive fenoldopam at 0.1 μg/kg/min following anesthesia induction to 72 hours postoperatively; the control group received standard therapy.90 There was a trend toward higher urine output and a fluid balance that was more negative in the fenoldopam group during the study period of 4 postoperative days, but this was not significantly different. Serum Cr was also not significantly different between the 2 groups. Hypotension rate and inotropic score were similar between the 2 groups. The authors hypothesized that the fenoldopam dose may have been too low to show clinical effect. The same group conducted a randomized, double-blinded, placebo-controlled study consisting of 80 infants undergoing CPB for cardiac repair surgery; high-dose fenoldopam (1 μg/kg/min) was used from CPB initiation to CPB weaning.91 The fenoldopam group’s urinary NGAL and CysC were significantly lower than for the control group immediately following the surgery. The incidence of AKI, as defined by pRIFLE, tended to be lower in the treatment group compared to the control group, 50% vs 72%, respectively (P = .08, OR 0.38; 95% CI 0.14, 1.02). Current evidence in the pediatric and neonatal literature does not support routine use of fenoldopam, but the results appear promising.
Maintenance Management of Intrinsic AKI
Fluid Management
It is important to maintain euvolemia in patients with AKI, particularly in the light of the recently emerging evidence that fluid overload is an independent mortality risk in critically ill children.92,93 The most helpful clinical tool in assessing fluid status is daily weight measurement. Infants with AKI should receive daily to twice daily weight measurements.3 Strict intake and output also need to be measured. A urethral Foley catheter is recommended to facilitate in strict urine output measurements.
Diuretics
Clinical studies in adult patients with AKI showed that diuretics do not reduce the need for RRT or the risk of hospital mortality.94 However, inducing urine output with diuretics can facilitate fluid balance in patients with oliguric AKI and, importantly in infants, enable the administration of more nutrition. The most commonly used diuretics include loop diuretics (furosemide, bumetanide) and thiazide diuretics (hydrochlorothiazide, metolazone). Loop diuretics decrease sodium reabsorption by inhibiting the Na-K-2Cl transporter on the thick ascending limb of the loop of Henle, thereby decreasing water reabsorption. Loop diuretics are highly protein bound and therefore minimally filtered at the glomerulus. Organic acid transporters located in the proximal tubule actively secrete loop diuretics from the blood into the urine, and they reach the Na-K-2Cl transporters in the loop of Henle.82 Hypoalbuminemia and the presence of other highly protein-bound drugs can decrease the tubular delivery of loop diuretics and lead to reduced diuretic effect.82 Loop diuretic dose requirement in AKI is higher than in the non-AKI setting because these diuretics depend on RBF for adequate delivery to the tubules. Furosemide bolus dose ranges from 0.5 to 1 mg/kg in the non-AKI setting; therefore, the initial bolus dose in the AKI setting should be at least 2 mg/kg.95 If furosemide induces diuresis in the patient, furosemide may be continued every 6–8 hours in term infants, every 12 hours in preterm infants older than 32 gestational weeks, and every 24 hours in preterm infants younger than 32 gestational weeks.95 Alternatively, the patient can be placed on continuous infusion starting at 0.1 mg/kg/h. Bumetanide is an alternate to furosemide. In a retrospective study consisting of 35 preterm infants with AKI, bumetanide (0.01–0.06 mg/kg/dose every 12–24 hours) increased urine output from 0.6 ± 0.6 mL/kg/h to 3.0 ± 2.1 mL/kg/h.96 Loop diuretics should be discontinued if adequate diuresis cannot be achieved following appropriately dosed diuretic trial to minimize the risk of adverse effects such as ototoxicity.82,95 Thiazide diuretics show a synergistic diuretic effect with loop diuretics by inhibiting the Na-Cl channel in the distal convoluted tubule, thereby blocking reabsorption distal to the loop diuretic’s site of action.82 If a patient remains oliguric despite adequate diuretic challenge, the patient’s fluid intake should be reduced to estimated insensible water loss plus output (urine output, stool output, ostomy output) to maintain fluid balance.
The infant’s renal function recovery may be accompanied by a polyuric diuretic phase, especially following the initial relief of obstruction, caused by the excretion of volume and solute retained during the renal obstruction. Prolonged polyuria may also occur due to the delayed recovery of tubular function relative to GFR, which results in excretion of excessive solute and water.97,98 Intravenous fluid may be needed during this phase to prevent the development of hypotension and prerenal AKI.
Hemodynamic Support
Patients with persistent hypotension despite adequate fluid resuscitation require systemic vasopressor support to minimize AKI. Current evidence in adult literature does not support the use of “renal-dose” or “low-dose” dopamine but emphasizes restoring BP in hypotensive patients to maintain end-organ perfusion and restore regional autoregulation.99–102 Although there are several controlled and uncontrolled clinical experimental studies of low-dose dopamine in infants, well-powered, randomized, controlled clinical trials are lacking, and there is not enough evidence to justify the routine use of low-dose dopamine for “renal protection.”103–106
Electrolyte Management
Hyponatremia, hyperkalemia, hyperphosphatemia, and metabolic acidosis can occur in AKI. Hyponatremia most often reflects hypervolemia and can be corrected with fluid restriction.3 Hyponatremia in the setting of polyuric AKI may reflect total body sodium depletion due to impaired tubular reabsorption; therefore, increased sodium intake is needed. Serum sodium less than 120 mEq/L may increase the infant’s risk of seizures, so correction with 3% saline should be considered.
Hyperkalemia may result secondary to decreased renal excretion due to reduced GFR and intracellular shift in AKI-induced metabolic acidosis. Hyperkalemia may result in cardiac arrhythmias and cardiac arrest; therefore, potassium should be monitored frequently in infants with AKI. Potassium should be removed from IVF and parenteral nutrition (PN) on identifying AKI to minimize the risk of hyperkalemia. Infants on formula should be placed on low-potassium nutrition such as maternal breast milk (MBM) or renal formula PM60/40. Depending on the degree of hyperkalemia, pharmaceutical interventions include (1) calcium chloride (via central line) or calcium gluconate to stabilize the cardiac resting membrane potential; (2) insulin/dextrose, sodium bicarbonate (if the patient is acidotic), and inhaled β-adrenergic agonist to shift potassium into the intracellular compartment; and (3) furosemide and sodium polystyrene sulfonate to remove potassium. Sodium polystyrene sulfonate (available as Kayexalate in the United States) is a cation exchange resin that binds potassium in exchange for sodium in the large intestine; therefore, hypernatremia can develop as a side effect. In addition, bowel necrosis has been reported in infants receiving sodium polystyrene sulfonate, particularly preterm infants, postoperative patients, patients with preexisting bowel obstruction, or patients receiving it administered as an enema.107,108 If hyperkalemia persists despite changing the formula to a renal formula, potassium in the formula can be further decreased by pretreating it with sodium polystyrene sulfonate, then decanting it.109–111
Hyperphosphatemia may occur due to decreased renal excretion in AKI. Hyperphosphatemia can be managed by restricting phosphorus intake by removing phosphate from PN and by decreasing enteral feed phosphorus content by pretreating then decanting it with phosphate binder such as sevelamer.112
Metabolic acidosis may occur in AKI due to reduced renal excretion of acid generated by dietary intake and cellular metabolism. It is important to consider serum ionized calcium and correct low calcium when considering treatment of metabolic acidosis. Total serum calcium is present in protein-bound form and free ionized form, which exerts physiologic effects. Acidosis treatment can shift ionized calcium to bind to protein, leading to an acute decrease in ionized calcium, which can precipitate tetany or seizures.27
Nutrition
Infants with AKI require optimal nutrition because AKI is often associated with catabolism, and malnutrition can develop quickly in these patients, further delaying their recovery.27 Enteral nutrition is preferred if the patient is stable enough to tolerate it. MBM is the preferred nutrition for infants with AKI. It may be helpful to fortify MBM to increase caloric intake. If MBM is not available, renal formula (PM60/40) is preferred in infants with hyperkalemia. Depending on the patient’s fluid status, PN should be concentrated as much as possible without compromising the administered calories and protein. Potassium, phosphorus, and magnesium may need to be removed from PN on initial diagnosis of AKI and returned in smaller amounts if needed.
Renal Replacement Therapy
Indications for initiating RRT in AKI include hyperkalemia or metabolic acidosis not responsive to pharmaceutical interventions, complications of hypervolemia, or the need for increased fluid intake with inadequate ability to increase diuresis to match the increased intake (eg, infants with liver failure who require high volumes of fresh-frozen plasma or infants who require more fluid for nutrition). Available RRT for infants with AKI includes peritoneal dialysis (PD), intermittent hemodialysis (IHD), and continuous hemofiltration with or without dialysis. The preferred type of RRT depends on patient factors as well as the availability of local personnel and equipment resources.
Peritoneal Dialysis
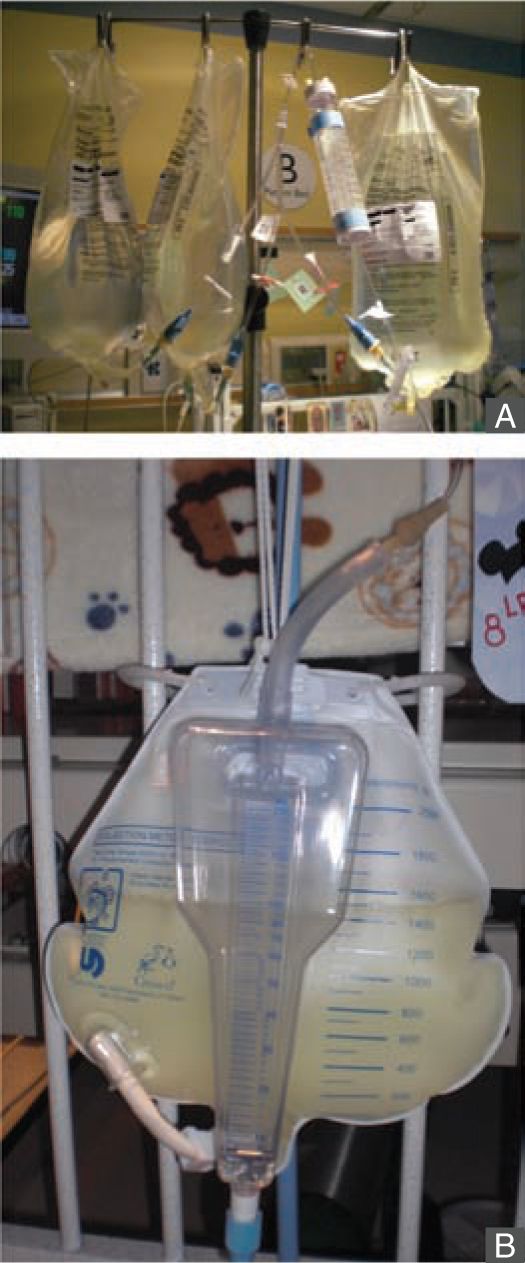
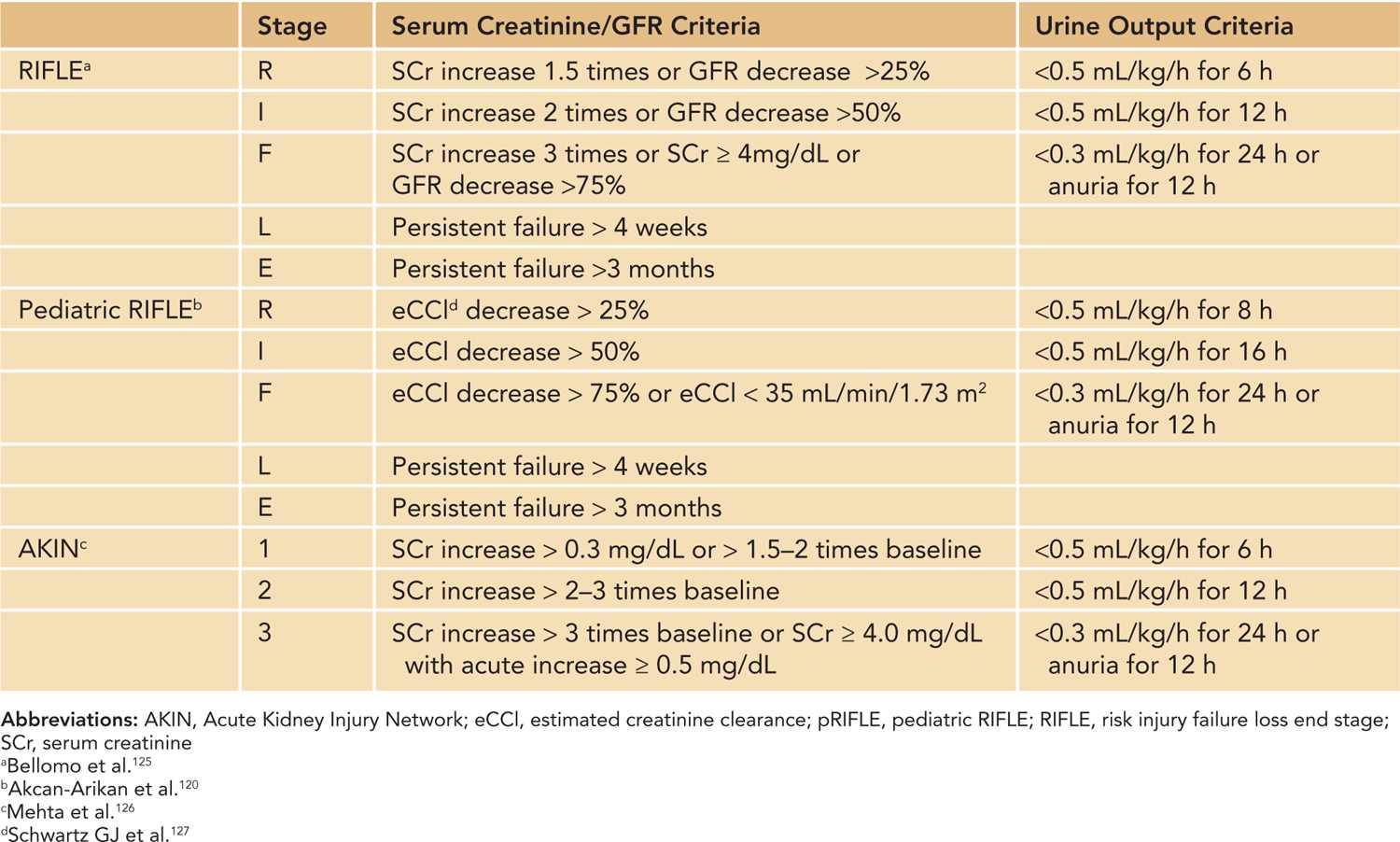
The PD system consists of peritoneal capillary blood flow (peritoneal microcirculation); the peritoneal membrane, which serves as the semipermeable dialysis membrane; and the dialysis fluid.113 Commercially available dialysis fluid contains an osmotic agent such as glucose or icodextrin, a buffer to correct the patient’s metabolic acidosis, and balanced concentrations of electrolytes sodium, calcium, and magnesium. The major driving force for ultrafiltration, the bulk movement of water across the semipermeable membrane, comes from the osmotic gradient provided by glucose or icodextrin in the dialysis fluid. Glucose concentrations of 1.5%, 2.5%, and 4.25% are commercially available, and various combinations of the dialysis fluid bags can be used at bedside to provide intermediate glucose concentrations, if needed. Solute exchange occurs primarily through diffusive transport driven by the solute concentration gradient and secondarily by solute removal by convection in ultrafiltration. The major advantages of PD include relatively easier access to the peritoneal cavity compared to vascular access, there is no need for systemic heparinization, and it is well tolerated in small infants and hemodynamically unstable patients (Figures 42-4 and 42-5). Disadvantages include slower correction of metabolic derangements and the risk of peritonitis, which is higher in those with a temporary compared to a permanent tunneled PD catheter. Additional assessment of risks and benefits are required in infants with intra-abdominal pathologies such as massive organomegaly and ostomies.27
FIGURE 42-4 Peritoneal dialysis is well tolerated in small infants. This infant was started on peritoneal dialysis soon after birth at 1.7 kg of birth weight.
FIGURE 42-5 Manual peritoneal dialysis setup in the neonatal intensive care unit. Manual peritoneal dialysis. A, Bureterol device (sterile graduated cylinder), Y set, and a graduated drain. Peritoneal dialysis bags consisting of different dextrose concentrations (1.5%, 2.5%, 4.25%) are attached to 1 end of the bureterol device, and the other end is attached to the patient’s peritoneal dialysis catheter via a Y set. B, The other limb of the Y set is connected to a graduated drain to measure the effluent.
Intermittent Hemodialysis
Solute exchange through the semipermeable dialyzer membrane occurs primarily through diffusion driven by the concentration gradient and by convective solute removal in the ultrafiltrate. Hemodialysis (HD) corrects metabolic derangements rapidly, and it can be used successfully in the initial management of hyperammonemia in urea cycle defects.114,115 Blood circuit priming is recommended when the extracorporeal volume exceeds 10% of the patient’s estimated blood volume (80 mL/kg × body weight) to prevent acute hypotension and anemia.113,116
Continuous Renal Replacement Therapy
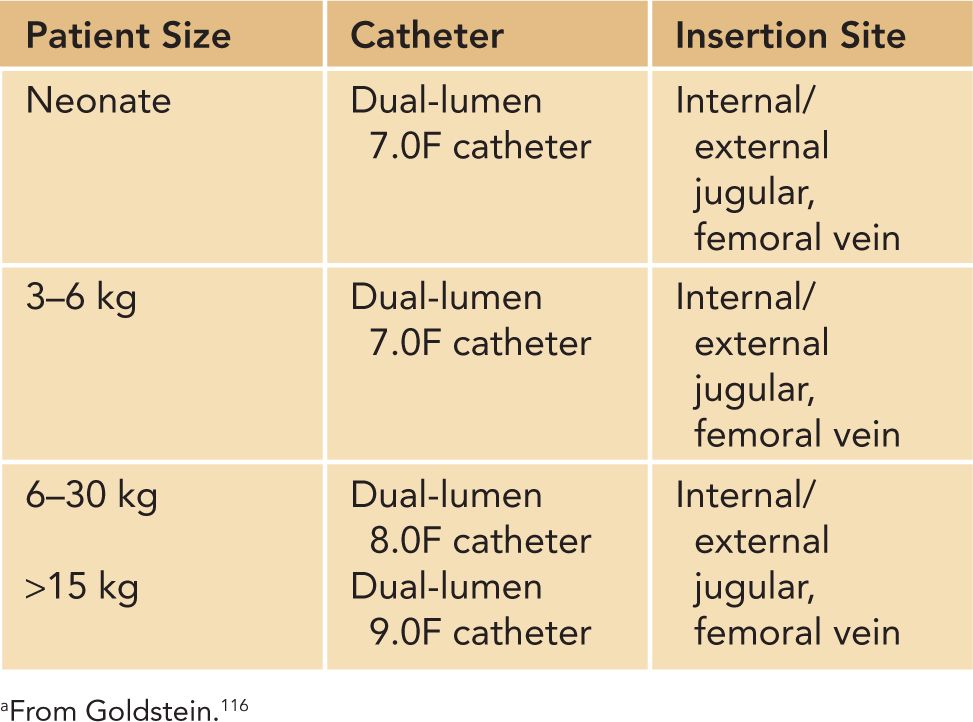
Continuous renal replacement therapy (CRRT) can be delivered as continuous venovenous hemofiltration (CVVH), which utilizes convective clearance; continuous venovenous hemodialysis (CVVHD), which utilizes diffusive clearance; and continuous venovenous hemodiafiltration (CVVHDF), which utilizes both diffusive and convective clearance. Ultrafiltration and solute clearance occur gradually over time; thus, this modality is well tolerated in hemodynamically unstable patients. CRRT can be used effectively in small infants, even in patients less than 3 kg.117 As with intermittent HD, blood circuit priming is necessary when the extracorporeal volume exceeds 10% of the patient’s blood volume. Good functional vascular access is required to deliver adequate CRRT and HD, and vascular access can be challenging in critically ill infants (Table 42-8). A double-lumen catheter can be placed in the internal jugular, femoral, or subclavian vein. The jugular vein is the preferred site as femoral vein catheters can experience complications from increased intra-abdominal pressure and subclavian catheters have been associated with stenosis at the subclavian-internal jugular junction.113 We do not use subclavian access for dialysis at our center. The optimal site of catheter placement, however, should be assessed by the patient characteristics, the risk of complications, and the operator’s skills and experience. Two 5F single-lumen catheters have been used to deliver CRRT, but we do not routinely recommend this method because the circuit survival has been shown to be poor at less than 20 hours.116,118
Table 42-8 Hemodialysis Catheter Size Based on Patient Sizea
Ethical Considerations
An extensive discussion of the ethical considerations in dialysis in neonates with end-stage renal disease (ESRD), such as ARPKD and anuric dysplastic kidneys, is beyond the scope of this chapter. The decision to initiate or withhold dialysis in a neonate with AKI requires a thoughtful evaluation of many factors, including the patient’s comorbidity, the risks and benefits of dialysis to the patient, and the parents’ wishes. Shooter et al discussed cases for which the parents and the medical team might consider withholding or withdrawing dialysis (Table 42-9) and outlined general guidelines to consider particularly when different parties disagree on what may be the best interest of the child (Table 42-10).119
Table 42-9 Situations for Which Withholding or Withdrawal of Medical Treatment Might Be Considereda