Background
Women with high levels of physical exercise have an increased demand for oxygen and nutrients. Thus, in pregnancies of women with high levels of exercise, it is conceivable that the supply of oxygen and nutrients to the placenta is suboptimal, and growth could be impaired.
Objective
The objective was to study the association of frequency of exercise during pregnancy with placental weight and placental to birthweight ratio.
Study Design
This was a prospective study of 80,515 singleton pregnancies in the Norwegian Mother and Child Cohort Study. Frequency of exercise was self-reported by a questionnaire at pregnancy weeks 17 and 30. Information on placental weight and birthweight was obtained by linkage to the Medical Birth Registry of Norway.
Results
Placental weight decreased with increasing frequency of exercise (tests for trend, P < .001). For nonexercisers in pregnancy week 17, the crude mean placental weight was 686.1 g compared with 667.3 g in women exercising ≥6 times weekly (difference, 18.8 g; 95% confidence interval, 12.0–25.5). Likewise, in nonexercisers in pregnancy week 30, crude mean placental weight was 684.9 g compared with 661.6 g in women exercising ≥6 times weekly (difference, 23.3 g; 95% confidence interval, 14.9–31.6). The largest difference in crude mean placental weight was seen between nonexercisers at both time points and women exercising ≥6 times weekly at both time points (difference, 31.7 g; 95% confidence interval, 19.2–44.2). Frequency of exercise was not associated with placental to birthweight ratio.
Conclusion
We found decreasing placental weight with increasing frequency of exercise in pregnancy. The difference in placental weight between nonexercisers and women with exercising ≥6 times weekly was small and may have no clinical implications.
Exercise increases the demand for oxygen and nutrients in working skeletal muscles. Thus, in pregnant women with high exercise levels, it is conceivable that the supply of oxygen and nutrients to the growing fetal-placental unit may be suboptimal. The growth of the fetal-placental unit could thereby be affected. Results from clinical and observational studies suggest that women with high levels of exercise during pregnancy give birth to offspring with slightly lower mean birthweight than women with low levels of exercise without increasing the risk of having offspring with birthweight below 2500 g or below the 10th percentile.
The placenta plays a key role in fetal growth and development. Except for 1 epidemiological study in Denmark, studies on the association of exercise during pregnancy with placental weight are based on small study samples, and the results are inconsistent. The findings in the Danish study suggest that placental weight may decrease with increasing frequency of exercise in early pregnancy (test for trend, P = .060). A disproportional size of the placenta relative to birthweight may indicate an unfavorable intrauterine environment for the fetus. Two small studies have addressed a possible association of exercise during pregnancy with placental weight relative to birthweight, but the results in these studies were conflicting.
Therefore, we studied whether the frequency of exercise in pregnancy is associated with placental weight or with placental weight relative to birthweight. We used information about exercise in pregnancy weeks 17 and 30 in a cohort of 80,815 women with a singleton pregnancy.
Materials and Methods
Study design, study population, and follow-up
During the years 1999–2008, all pregnant women scheduled to give birth at 50 hospitals in Norway were targeted for recruitment to the Norwegian Mother and Child Cohort Study ( www.fhi.no/morogbarn ). The women were recruited at a routine fetal ultrasonographic examination in pregnancy weeks 17–19. This examination is part of the public antenatal health care program, offered free of charge to all pregnant women living in Norway.
The Norwegian Mother and Child Cohort Study had no exclusion criteria, and 40.6% of all eligible women agreed to participate. The present study is based on version 8 of the quality-assured data files, released for research in 2014.
Data were obtained through 2 self-administered questionnaires, distributed and returned by postal mail. The first questionnaire was completed during the second trimester (mean 17.4 weeks, SD 2.2 weeks) and included questions about sociodemographic factors, general health, reproductive history, and leisure time exercise. The second questionnaire was completed during the third trimester (mean 30.6 weeks, SD 1.5 weeks) and included questions about maternal health and leisure time exercise.
Information about placental weight, birthweight, and other pregnancy outcomes was obtained by linkage to the Medical Birth Registry of Norway. This registry contains information about all births in Norway after pregnancy week 16 as obtained by compulsory notification by the midwife or the doctor attending the delivery.
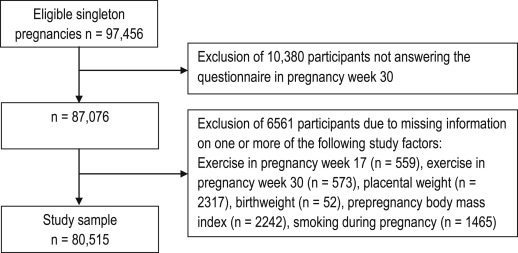
Of the women who agreed to participate in the Norwegian Mother and Child Cohort Study, 94.9% returned the first questionnaire and 91.0% returned the second questionnaire. Women with a singleton pregnancy, and who had answered both questionnaires, were eligible for our data analyses, a total of 87,076 women. We excluded 6561 women with missing information on 1 or more of the following study factors: exercise in pregnancy week 17 (559 missing), exercise in pregnancy week 30 (573 missing), placental weight (2317 missing), birthweight (52 missing), prepregnancy body mass index (BMI) (2242 missing), and smoking during pregnancy (1465 missing), leaving 80,515 singleton pregnancies to our study sample ( Figure ).
Study factors
Placental weight in grams was our main outcome measure. The newborn and the placenta were routinely weighed shortly after birth. According to Norwegian standardized routines, the placentas were placed in a bowl immediately after birth and weighed fresh with membranes and umbilical cord attached, independent of delivery mode. The weight of the bowl was subtracted from the sum weight. The placental to birthweight ratio was calculated as placental weight (grams) divided by birthweight (grams).
The main exposure variables were frequency of leisure time exercise in pregnancy weeks 17 and 30. In the questionnaires, the participants reported how often they performed the following exercises: strolling, brisk walking, running (jogging or orienteering), bicycling, training in a fitness center, swimming, aerobics (low or high impact), prenatal aerobic, dancing, cross-country skiing, ball games, horseback riding, and other exercises.
Based on the definition of exercise by Caspersen et al, strolling was categorized as nonexercise. We combined all exercise frequencies (excluding strolling) into 1 variable categorized as the following: never, 1–3 times monthly, 1–2 times weekly, 3–5 times weekly, and ≥6 times weekly. Nonexercisers were those who responded never to all exercises listed or who reported strolling only. We also combined the frequency of exercise at pregnancy weeks 17 and 30 into 1 variable, and we categorized the frequency at both time points as follows: never, <3 times weekly, ≥3 times weekly, and ≥6 times weekly. Switch in frequency of exercise from week 17 to week 30 was defined as a change in frequency.
The associations of type of exercise with placental weight were investigated in the supplemental analyses. We grouped the type of exercise as follows: no exercise (strolling or none), brisk walking, non–weight-bearing exercise (cycling or swimming), low-impact exercise (prenatal aerobics, low-impact aerobics, dancing, cross-country skiing, or fitness training), high-impact exercise (running, jogging, orienteering, or ballgames), and other exercise (horseback riding or other). A mixed exercise group included women without a single dominant type of exercise (for example, 1 session of jogging and 1 session of swimming weekly).
We identified potentially confounding factors by using directed acyclic graphs and assessed factors that may be associated with leisure-time exercise and with placental weight. Values for confounding factors were obtained from the questionnaires: BMI (kilograms per square meter) before pregnancy based on self-reported weight and height, cigarette smoking during pregnancy (yes/no), nausea with vomiting before pregnancy week 17 (yes/no), and years of education (<13 years, 13–16 years, ≥17 years, and missing). Additionally, we obtained information from the Medical Birth Registry of Norway on maternal age (years), parity (para 0, para 1, and para ≥2), maternal diabetes (including diabetes type 1, type 2, and gestational diabetes) (yes/no), preeclampsia (yes/no), and hemoglobin concentrations <9 g/dL in pregnancy (yes/no).
Statistical methods
The associations of exercise frequency and type with placental weight and with placental to birthweight ratio were estimated as crude and adjusted unstandardized beta coefficients with 95% confidence intervals (CI) by applying linear regression analyses. The beta coefficients can be interpreted as a change in placental weight (grams) by a change in the category of exercise frequency. Nonexercisers were used as the reference group. We performed separate analyses in women with a BMI <25 kg/m 2 and in women with a BMI ≥25 kg/m 2 and also in primiparous and in multiparous women. We used the general linear model with polynomial contrast to assess trends in mean placental weight and placental to birthweight ratio according to the increasing frequency of exercise.
In additional analyses, we also studied the association of exercise frequency with the odds of having placental weight among the 5% smallest using logistic regression analyses, and we used the nonparametric trend test (nptrend) to assess trends according to the increasing frequency of exercise. We used the statistical software packages IBM SPSS version 20.0 (IBM Corp, Armonk, NY) and STATA 14 SE (StataCorp, College Station, TX) for the statistical analyses.
Ethical considerations
The Norwegian Mother and Child Cohort Study was approved by the Regional Committee for Medical and Health Research Ethics (S-97045; S-95113) and by the Norwegian Data Protection Authority. All participants signed an informed consent form. This particular study is approved by The Norwegian Mother and Child Cohort Study Steering Committee at the Norwegian Institute of Public Health (PDB 1595; reference 14/1185).
Results
Mean maternal age was 30.2 years (SD 4.5 years), 45.8% were first-time mothers, and in 68.6% of the women the prepregnancy BMI was <25 kg/m 2 . Overall, the mean placental weight was 680.1 g (SD 197.0 g), and the mean birthweight was 3607.1 g (SD 541.5 g) ( Table 1 ).
| Characteristics | n, % | Mean (SD) |
|---|---|---|
| Maternal age, y | 30.2 (4.5) | |
| Prepregnancy body mass index, kg/m 2 | 24.1 (4.3) | |
| Parity | ||
| Para 0 | 36,880 (45.8) | |
| Para 1 | 28,461 (35.3) | |
| Para ≥ 2 | 15,174 (18.8) | |
| Maternal diabetes | 1158 (1.4) | |
| Preeclampsia | 2873 (3.6) | |
| Hemoglobin <9 g/dL | 289 (0.4) | |
| Nausea with vomiting | 29,373 (36.5) | |
| Educational level, y | ||
| ≤12 | 26,125 (32.4) | |
| 13–16 | 31,943 (39.7) | |
| ≥17 | 18,458 (22.9) | |
| Missing | 3989 (5.0) | |
| Smoking during pregnancy | 6618 (8.2) | |
| Married /cohabitant | 77,493 (96.2) | |
| Gestational length, wks a | 39.5 (1.7) | |
| Birthweight, g | 3607.1 (541.5) | |
| Placental weight, g | 680.1 (197.0) | |
| Placental to birthweight ratio | 0.1893 (0.0496) |
In pregnancy week 17, 22.4% of the women never took part in leisure-time exercise, and in pregnancy week 30 the proportion of nonexercisers was 32.7%. A total of 13.3% of the women were nonexercisers at both time points ( Table 2 ). The corresponding proportions of women who exercised ≥6 times weekly were 5.0%, 2.9%, and 1.3%.
| Exercise frequency during pregnancy | Pregnancies, n, % | Placental weight, g | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Crude B (95% CI) | Test for trend | Adjusted B a (95% CI) | Test for trend | ||
| Pregnancy week 17 | ||||||
| Never (reference) | 18,008 (22.4) | 686.1 (189.7) | 0.0 | P < .001 | 0.0 | P = .079 |
| 1–3 times monthly | 15,975 (19.8) | 683.9 (194.1) | –2.2 (–6.4 to 2.0) | –0.7 (–4.9 to 3.5) | ||
| 1–2 times weekly | 23,683 (29.4) | 681.2 (216.0) | –4.9 (–8.7 to –1.1) | –0.3 (–4.1 to 3.6) | ||
| 3–5 times weekly | 18,852 (23.4) | 672.7 (183.7) | –13.3 (–17.4 to –9.3) | –3.9 (–8.0 to 0.2) | ||
| ≥6 times weekly | 3997 (5.0) | 667.3 (182.0) | –18.8 (–25.5 to –12.0) | –3.6 (–10.4 to 3.2) | ||
| Pregnancy week 30 | ||||||
| Never (reference) | 26,315 (32.7) | 684.9 (187.5) | 0.0 | P < .001 | 0.0 | P = .036 |
| 1–3 times monthly | 15,486 (19.2) | 682.7 (181.4) | –2.2 (–6.1 to 1.7) | 0.9 (–3.0 to 4.8) | ||
| 1–2 times weekly | 21,581 (26.8) | 680.4 (224.0) | –4.5 (–8.0 to –1.0) | 2.0 (–1.6 to 5.6) | ||
| 3–5 times weekly | 14,802 (18.4) | 671.6 (193.2) | –13.2 (–17.2 to –9.3) | –1.7 (–5.8 to 2.3) | ||
| ≥6 times weekly | 2331 (2.9) | 661.6 (152.1) | –23.3 (–31.6 to –14.9) | –5.9 (–14.3 to 2.4) | ||
| Combined weeks 17 and 30 | ||||||
| Never (reference) | 10,710 (13.3) | 685.7 (180.2) | 0.0 | P < .001 | 0.0 | P = .020 |
| <3 times weekly | 22,213 (27.6) | 682.0 (214.8) | –3.7 (–8.2 to 0.9) | 2.0 (–2.6 to 6.5) | ||
| Change in frequency b | 36,843 (45.8) | 681.5 (195.3) | –4.1 (–8.4 to 0.1) | 1.7 (–2.6 to 5.9) | ||
| ≥3 times weekly | 9698 (12.0) | 667.2 (183.2) | –18.4 (–23.9 to –13.0) | –3.8 (–9.3 to 1.8) | ||
| ≥6 times weekly | 1051 (1.3) | 654.0 (140.5) | –31.7 (–44.2 to –19.2) | –10.9 (–23.4 to 1.5) | ||
a Adjusted for prepregnancy body mass index (kilograms per square meter), parity, diabetes, preeclampsia, maternal age, hemoglobin <9 g/dL, nausea with vomiting before pregnancy week 17, education level, and smoking during pregnancy
b Switching from exercising <3 times weekly to ≥3 times weekly or vice versa from week 17 to week 30 or switching from never at 1 time point to exercising at any frequency at the other time point.
Crude mean placental weight decreased by increasing frequency of exercise in pregnancy week 17, and the crude mean decrease in placental weight from nonexercisers to women exercising ≥6 times weekly was 18.8 g (95% CI, 12.0–25.5) (test for trend, P < .001) ( Table 2 ).
A similar pattern was observed for exercise in pregnancy week 30, and the crude decrease in the mean placental weight from nonexercisers to women exercising ≥6 times weekly was 23.3 g (95% CI, 14.9–31.6) (test for trend, P < .001). Women who exercised ≥6 times weekly in pregnancy week 17 and in pregnancy week 30 had 31.7 g (95% CI, 19.2–44.2) lower placental weight than nonexercising women (test for trend, P < .001).
Overall, the lowest mean placental weight was observed in women who exercised ≥6 times weekly in both pregnancy week 17 and pregnancy week 30, followed by women who exercised ≥6 times weekly in pregnancy week 30 and women who exercised ≥6 times weekly in pregnancy week 17 ( Table 2 ).
After adjustment for the potentially confounding factors listed in the previous text, the associations of exercise frequency in pregnancy with a placental weight were attenuated, and the decreasing trend was no longer statistically significant for exercise frequency in pregnancy week 17 ( Table 2 ).
Prepregnancy BMI and parity were the main confounding factors. In separate analysis in women with a BMI <25 kg/m 2 and in women with a BMI ≥25 kg/m 2 , as well as in primiparous and multiparous women, the trend of decreasing placental weight with increasing frequency of exercise was still present ( Table 3 ). Again, adjustment for other study factors attenuated the associations. However, a statistically significant inverse trend was present in women with a BMI <25 kg/m 2 (test for trend, P = .003) and in primiparous women (test for trend, P = .037).
| Exercise frequency at pregnancy week 30 | Pregnancies, n, % | Placental weight, g | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Crude B (95% CI) | Test for trend | Adjusted B a (95% CI) | Test for trend | ||
| Prepregnancy BMI <25 kg/m 2 (n = 55,194) | ||||||
| Never (reference) | 16,341 (29.6) | 670.4 (176.2) | 0.0 | P < .001 | 0.0 | P = .003 |
| 1–3 times monthly | 10,172 (18.4) | 668.3 (176.3) | –2.1 (–6.8 to 2.5) | –0.9 (–5.5 to 3.8) | ||
| 1–2 times weekly | 15,201 (27.5) | 668.7 (207.2) | –1.7 (–5.9 to 2.4) | 0.5 (–3.7 to 4.7) | ||
| 3–5 times weekly | 11,477 (20.8) | 663.9 (192.8) | –6.5 (–11.0 to –2.0) | –3.2 (–7.7 to 1.4) | ||
| ≥6 times weekly | 2003 (3.6) | 656.0 (150.0) | –14.4 (–23.1 to –5.7) | –9.7 (–18.5 to –0.9) | ||
| Prepregnancy BMI ≥ 25 kg/m 2 (n = 25,321) | ||||||
| Never (reference) | 9974 (39.4) | 708.6 (202.5) | 0.0 | P = .127 | 0.0 | P = .429 |
| 1–3 times monthly | 5314 (21.0) | 710.2 (187.7) | 1.6 (–5.5 to 8.7) | 3.0 (–4.1 to 10.0) | ||
| 1–2 times weekly | 6380 (25.2) | 708.2 (257.5) | –0.4 (–7.1 to 6.3) | 2.1 (–4.6 to 8.9) | ||
| 3–5 times weekly | 3325 (13.1) | 698.3 (192.1) | –10.3 (–18.7 to –2.0) | –6.0 (–14.4 to 2.4) | ||
| ≥6 times weekly | 328 (1.3) | 696.0 (160.6) | –12.6 (–36.1 to 10.8) | –6.1 (–29.5 to 17.3) | ||
| Primiparous women, para 1 (n = 36,880) | ||||||
| Never (reference) | 9539 (25.9) | 668.0 (193.7) | 0.0 | P < .001 | 0.0 | P = .037 |
| 1–3 times monthly | 6579 (17.8) | 666.7 (174.0) | –1.3 (–7.4 to 4.7) | 0.3 (–5.7 to 6.3) | ||
| 1–2 times weekly | 10,768 (29.2) | 665.4 (210.8) | –2.6 (–7.9 to 2.7) | 0.1 (–5.3 to 5.4) | ||
| 3–5 times weekly | 8428 (22.9) | 662.2 (205.6) | –5.9 (–11.5 to –0.2) | –0.4 (–6.1 to 5.3) | ||
| ≥6 times weekly | 1566 (4.2) | 650.6 (138.7) | –17.4 (–27.7 to –7.2) | –9.8 (–20.1 to 0.5) | ||
| Multiparous women, ≥para 2 (n = 43,635) | ||||||
| Never (reference) | 16,776 (38.4) | 694.5 (193.7) | 0.0 | P = .042 | 0.0 | P = .606 |
| 1–3 times monthly | 8907 (20.4) | 694.5 (185.8) | 0.0 (–5.1 to 5.2) | 1.2 (–4.0 to 6.3) | ||
| 1–2 times weekly | 10,813 (24.8) | 695.3 (235.5) | 0.8 (–4.0 to 5.6) | 3.5 (–1.4 to 8.3) | ||
| 3–5 times weekly | 6374 (14.6) | 684.2 (174.6) | –10.3 (–16.1 to –4.5) | –4.3 (–10.1 to 1.4) | ||
| ≥6 times weekly | 765 (1.8) | 684.2 (174.3) | –10.3 (–24.8 to 4.3) | –0.1 (–14.6 to 14.3) | ||
a Adjustments for diabetes, preeclampsia, maternal age, hemoglobin <9 g/dL, nausea with vomiting before pregnancy week 17, educational level, and smoking during pregnancy; additional adjustment in prepregnancy BMI strata was parity, whereas additional adjustment in parity strata was prepregnancy BMI.
Frequency of exercise in pregnancy was also positively associated with the risk (crude odds ratio) of having a placenta among the 5% smallest (tests for trend, P < .01) ( Table 4 ). The associations were attenuated after adjusting for the other study factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


