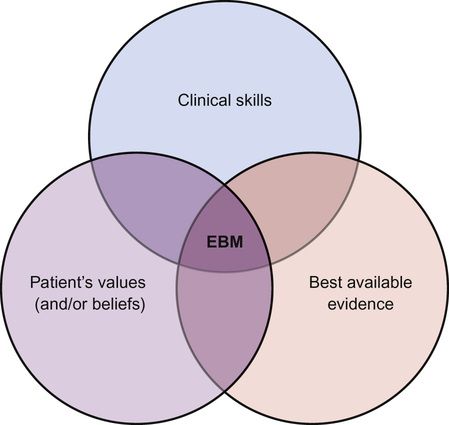
Bob Phillips, Peter Cartledge Evidence-based medicine (EBM) has now established itself as a key principle at the heart of modern clinical life. But what is it? In this chapter we will look at the principles of evidence-based medicine and how to practise it. When we make a clinical decision (e.g. should I give this wheezy child a nebulized or spaced β2 agonist?), we need to think about the patient and the overall outcome. There could be beneficial outcomes, but these should be weighed against the possibility of negative effects. As clinicians, we instinctively assess the chances of these outcomes, weigh them, and conclude on a course of action. If we are treating a child with an acute exacerbation of asthma, we may want to know what is the best mode of delivery for a β2 agonist? But what does best mean? Patient satisfaction? Ease of delivery? Fewer symptoms? Fewest side effects? Fewer admissions? Most cost-effective? Least expensive? For the clinician, the process of practising EBM can be difficult, time consuming, and (dare we say it) boring. In this chapter, these barriers will be tackled with examples of EBM in practice, revealing that the five minutes spent thinking this through may have saved you hours of work whilst improving the care provided to your patients. EBM was defined by one of the founders of the EBM movement, David Sackett, as ‘the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients’. But what does that mean in real terms? EBM is a shorthand term for five linked ideas: 1. Our practice should be a meeting of (Fig. 39.1): – Our clinical skills (including history, examination and diagnosis building) – Our patient’s own values, preferences and beliefs 5. Clinicians, including those in training, should continuously evaluate their performance. Some misconceptions about EBM are listed in Box 39.1. Practicing EBM improves patient care. There really should be no other incentive. Considering this in terms of the four pillars of medical ethics can be helpful: There are also selfish reasons for wanting to practise EBM: There are 11 new systematic reviews and 75 new trials published every day (Bastian 2010). It is impossible to keep up-to-date with all medical advancements, so long-term professional strategies are required to meet our learning and information needs. Reading this book is an example of just in case information: packing in a range of general nuggets to provide a good underlying understanding of paediatrics in order to be good clinicians. When taken to the extreme, it is the diligent, regular reading of a series of journals ‘just in case’ a particular case was to present. This is inefficient and induces guilt when you cannot manage it. This is a lifelong skill – the acquisition of information ‘just’ as you need it, e.g. reading a patient’s clinic notes before they arrive. In the EBM context, it is identifying information that will help us best manage our patients by seeking rapid answers to specific queries. Research has shown that a doctor in training will have two unanswered questions for every three patients they consult (Green et al 2000). An inquisitive clinician could have many more questions. As our clinical experience improves, this number is unlikely to change, but the content may become more focused. The practice of EBM is a multi-step process. Each of these steps (Table 39.1) requires individual skills and practice, though some resources will allow us to shortcut some of these steps. Throughout this chapter, there are examples of the ‘five steps’ in practice (see also Tables 39.3, 39.4, 39.6 and 39.9). It is all too easy to practise medicine without asking questions, as asking questions exposes potentially embarrassing gaps in our knowledge. The first step of EBM is to address this challenge and admit ignorance or uncertainty, then convert our information needs into answerable questions. This means having an inquisitive mind. Looking at the anatomy of enquiry, ask initially ‘What sort of question am I asking?’ If it is a clinical question then it can be grossly categorized as ‘foreground’ or ‘background’. Background questions are broad, and are often ‘what is’ or ‘what causes’ type questions, e.g. ‘What causes asthma in childhood?’ Foreground questions are specific and pointed, and can be fitted into a ‘PICO’ framework (patient-problem, intervention, comparison, outcome). This art is known as ‘framing a clear question’ and is an essential skill. A well-framed question must be directly related to the patient and structured in order to search for a relevant and precise answer. A popular method for framing a clinical question is the PICO method (Box 39.2): Choosing the right outcomes to search is incredibly important. If you have a strong opinion on a topic, it is likely to be drawn from experience, e.g. always giving inhaled salbutamol to wheezy children in A&E. But what do we actually want to know about our intervention? Will it stop the patient dying? Will it keep them out of hospital for longer? Will they feel better? These are all important questions. The aim of this step is the acquisition of good quality information/evidence to answer your skilfully constructed question. This can be difficult, but with practice and a few tips on where to look, it gets easier. The process therefore follows three steps: 1. Converting your PICO question into searchable terms 2. Searching for secondary sources (e.g. guidelines, Cochrane/DARE, etc.) 3. Finding primary sources if secondary sources are not available (e.g. PubMed). (See http://www.youtube.com – search for ‘PubMed Advanced Search Builder’ in YouTube for a 3-minute tutorial on how to search in PubMed.). Getting your search right is both an art and a science. A good search is both sensitive and specific. A sensitive search will not miss any relevant papers, a specific search will not have too many irrelevant articles. Once you have written a sound PICO question you must convert the question into searchable terms. To form a search strategy: 2. Search for the correct MeSH term (if you are using a database that does not automatically map). ‘MeSH’ is essentially the National Institute of Health (NIH) thesaurus of medical terms that guides towards the ‘correct medical term’. This means a searching clinician is able to find the relevant research, including when the papers’ authors have not used the ‘preferred’ medical term). (See http://www.nlm.nih.gov/mesh/; Tutorial: http://www.nlm.nih.gov/bsd/viewlet/mesh/searching/mesh1.html). 3. Combine the search terms using the correct Boolean operators (Table 39.2). Table 39.2 Boolean operators There is probably no correct answer to the question, ‘where is best to search?’ You are likely to find databases which you are more comfortable using. Trip®, the Cochrane library® or PubMed® are good databases to be familiar with (Box 39.3). A hierarchical search aims to look for the best quality evidence first, and then work downwards if insufficient research is discovered (Fig. 39.2). If you are searching in a clinical environment, a database such as Trip® will often find results quickly (Table 39.3) and present them in evidence type. If this fails, the Cochrane library and DARE website should be searched (Table 39.4). If this does not yield any results, then PubMed’s ‘Clinical Queries’ is the next best port of call followed by a search for primary resources in PubMed. Table 39.4 Best evidence topic (BET) – diagnosis Scenario: You start at a new hospital where you undertake ‘postnatal checks’ on newborn infants. You notice that in this hospital you do not need to perform post-ductal pulse oximetry testing. You discuss this with your consultant, who asks you to find out more exact details on the benefits of this. Step 1: Asking a question (PICO): In an asymptomatic newborn infant [patient], does post-ductal pulse oximetry [intervention] increase the number of infants correctly identified with congenital heart disease or reduce mortality rates [outcomes]? Step 2: Acquiring evidence: Search terms: (Infant, newborn OR infant* OR newborn OR ‘newborn infant’ OR neonat*) AND (heart defects, congenital OR congenital heart defect* OR Defect*, congenital heart OR heart, malformation of OR heart abnormalit* OR congenital heart disease OR cyanotic heart disease OR cyanotic heart defect OR congenital heart malformation) AND (oximetry OR oximetry, pulse OR blood gas monitoring, transcutaneous OR oximetry, transcutaneous OR oximetry, transcutaneous OR saturation*, oxygen OR oxygen saturation*) Databases: Cochrane: 164 results, 4 non-Cochrane reviews including below meta-analysis. Step 3: Appraising the evidence: 13 eligible studies with data for 229,421 asymptomatic newborn babies. 118 infants with critical congenital heart defects. 748 false positives. 33 false negatives. Pulse oximetry. 60% of studies used foot alone (postductal). Sensitivity 76.5% (95% CI 67.7–83.5), specificity was 99.9% (99.7–99.9), false positive rate 0.14 (0.06–0.33). Likelihood ratio positive 549 (238–1195), likelihood ratio negative 0.24 (0.17–0.33). Lower false positive rate if oximetry >24 hours (p=0.0017), but no effect on sensitivity. Clear description of search strategy. No statistical description of heterogeneity. Significant publication bias was reported. Commentary: Screening for critical congenital heart defects in newborn babies can aid early recognition, with the prospect of improved outcome. In this case, the new doctor was not interested in an individual patient but a population. As the search had the potential to lead to widespread change in the clinical assessment of all newborns, the search for evidence needed to identify the most relevant and highest quality available. The search was therefore very comprehensive. Though this systematic review found that pulse oximetry is highly specific for critical congenital heart disease, it does not look at broader outcomes. For example, do infants who have their diagnosis made earlier using screening have better long-term outcomes (e.g. mortality)? This would be an important consideration when balancing the cost (equipment, time, etc.) of implementing such a screening tool. Step 4: Applying the evidence (the clinical bottom line):
Evidence-based paediatrics
Introduction
What is EBM?
Why practise EBM?
Altruistic reasons for practising EBM
Personal reasons for practising EBM
Meeting our information needs
‘Just in case’ information
‘Just in time’ information
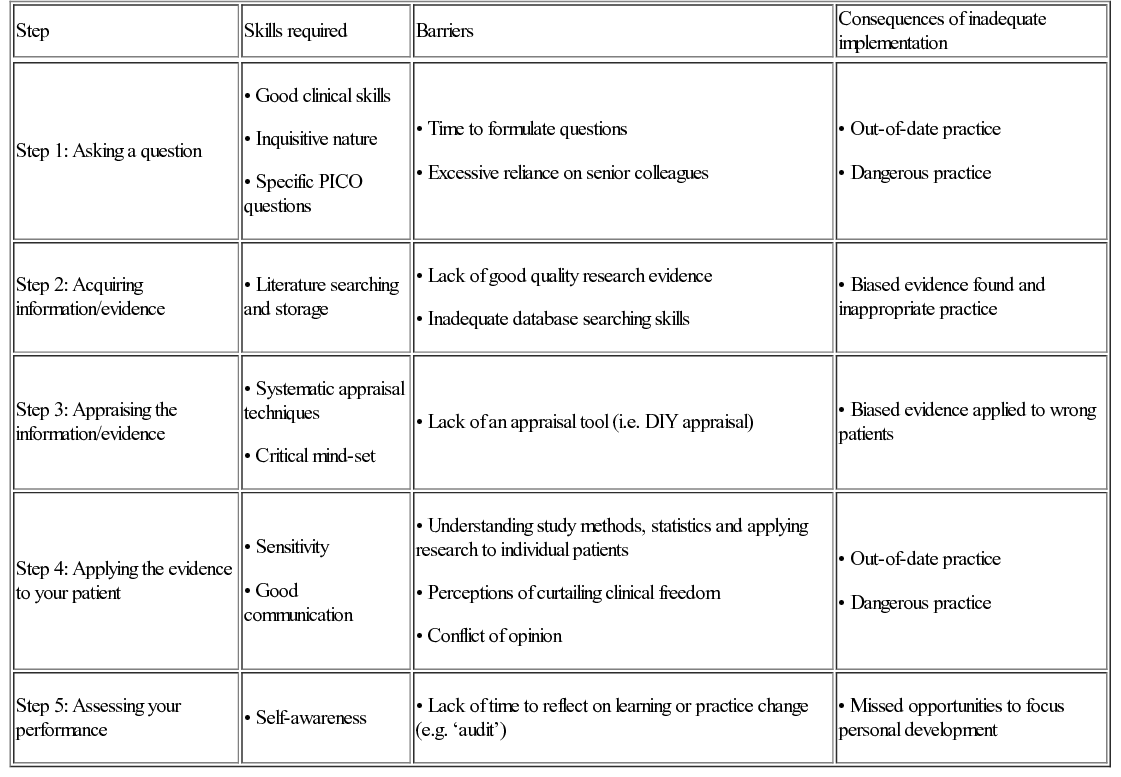
The five steps of EBM
Step 1: Asking a question
Framing a clear question: PICO questions
Step 2: Acquiring information/evidence
Converting PICO questions into searchable terms
Term
Search description
OR
(Infant OR child) will find all articles/documents containing at least one of these keywords
NOT
(Infant NOT child) will find articles/documents containing the keyword infant but exclude those also including the keyword child
AND
(Infant AND child) will find articles/documents containing both of these keywords
* (Truncation)
Infant* will find articles/documents containing the keywords infant OR infants OR infantile OR infancy, etc.
Where to search
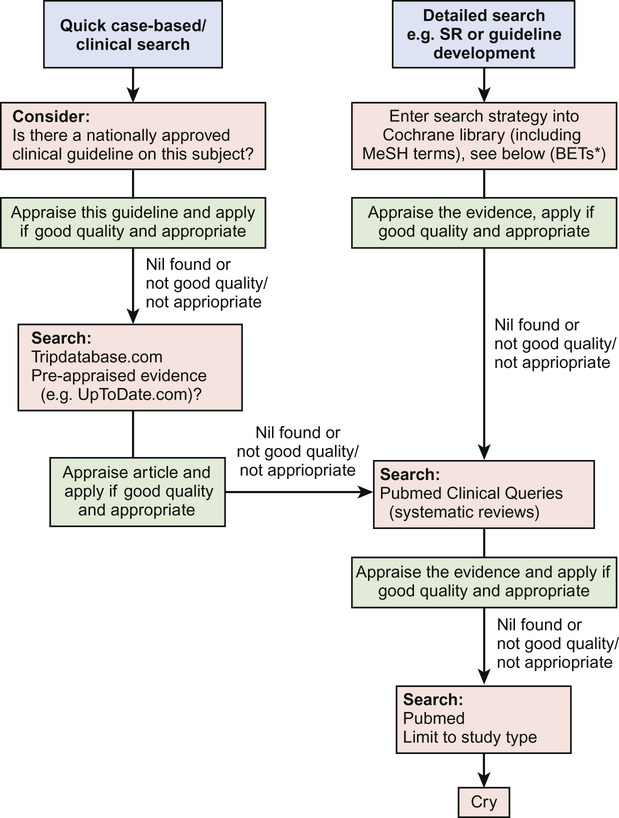
Hierarchical searches
Diagnosis – cyanotic heart disease
Study group
Intervention
Study type
Outcomes
Key results
Comments
Systematic review and meta-analysis of 12 cohort and 1 case-control study.
Detection of critical congenital heart defects.
Get Clinical Tree app for offline access

Evidence-based paediatrics
Chapter 39
Learning objectives
By the end of this chapter the reader should: