Epilepsy Treatment: Antiepileptic Drug Therapy
Blaise F. D. Bourgeois
Although other treatment modalities, such as the ketogenic diet, epilepsy surgery, and the vagus nerve stimulator, are appropriate options at some point for certain patients, antiepileptic drugs (AEDs) represent almost invariably the first and, in the great majority of patients, the only treatment in patients diagnosed with epilepsy. Until 1993, 4 AEDs represented more than 90% of all prescriptions for epilepsy: phenobarbital, phenytoin, carbamazepine, and valproate. These drugs are now referred to as the “older” AEDs. Adrenocorticotropic hormone (ACTH) was available but was used only for limited specific indications. Since 1993, many “newer” AEDs have become available. As a group, most of these newer drugs offer welcome alternatives with less or different side effects, fewer or no pharmacokinetic interactions, and a spectrum of efficacy that covers a wider range of different seizure types. However, despite the availability of these newer drugs, the percentage of patients whose seizures can never be fully controlled by medication has not been reduced and remains around 25% to 30% of all patients diagnosed with epilepsy. With so many AEDs to choose from, the challenge of matching the best possible drug to a given patient has grown substantially. The first goal of therapy is to find as rapidly as possible the AED that will provide the best possible seizure control while maximizing tolerability and safety. The currently available evidence-based guidelines address only a small proportion of the seizure types and syndromes, and selecting the most appropriate drug will also involve clinical experience and art.1,2 Often the choice will have to be tailored for a given patient, and in addition to seizure type and epilepsy syndrome, personal characteristics of the patient will have to be taken into account, such as gender, age, comorbid conditions, other medications, and lifestyle.
GENERAL PRINCIPLES OF THERAPY
 HOW DOES EPILEPSY DIFFER IN CHILDREN?
HOW DOES EPILEPSY DIFFER IN CHILDREN?
Compared to practice in adults, the drug treatment of epilepsy in children differs in many ways. Children have seizure types, epilepsy syndromes, and underlying etiologies that cover a much broader spectrum. As the brain matures, seizures in children may evolve over time. The decision to initiate treatment after a first or even second seizure may differ in children, due to factors such as prognosis and impact of seizures on lifestyle. Antiepileptic therapy in children occurs in a context of growing, learning, and developing. Children may receive therapies that are only exceptionally used in adults, such as adrenocorticotropic hormone (ACTH), steroids, or the ketogenic diet. Often, antiepileptic drugs (AEDs) are used off label in children, either based on age or based on seizure type. Certain side effects of medications are more likely to occur in the pediatric age range, whereas others are more likely to occur in adults. Because children need to develop and learn, possible cognitive side effects of AEDs are a particular concern in this age group.3 Children’s pharmacokinetics differ, because they have invariably shorter drug elimination half-lives and higher clearances, which translate into substantially higher dosage requirements (in mg/kg/day). A subgroup of children has epilepsies that are likely to remit, and children as a group are less likely to experience seizure relapse when AEDs are tapered and discontinued following successful treatment. All these differences can determine drug choice, dosage, and monitoring of therapy. Also, the goal of AED therapy is to fully control seizures with no or minimal side effects, but the goal of epilepsy treatment is broader, because it is to help the child and the family to deal not only with the seizures, but also with their medical and psychosocial consequences, and with the patient’s other possible neurological comorbidities. Examples of comorbid conditions include mental retardation, cerebral palsy, attention deficit, depression, hydrocephalus, and metabolic disorder. Over the past decades, the treatment of epilepsy has evolved from a seizure control emphasis to a quality of life emphasis.
 DECISION TO INITIATE OR WITHHOLD PROPHYLACTIC TREATMENT
DECISION TO INITIATE OR WITHHOLD PROPHYLACTIC TREATMENT
In a child with a single or several new-onset unprovoked epileptic seizures, the first task is to determine whether daily prophylactic AED therapy is the best option, or whether it would be preferable to withhold treatment for the time being. The following questions should be addressed: What is the risk of seizure recurrence? What would be the likely or possible consequences of a seizure recurrence? Does early treatment influence outcome? What are the risks associated with treatment? What are the patient’s age, occupation, and preference? The question of the risk of seizure recurrence in children following a first unprovoked seizure has been addressed in many studies, and a meta-analysis of 16 studies provides very useful numbers4:
 RISK OF RECURRENCE AT 2 YEARS
RISK OF RECURRENCE AT 2 YEARS
The risk of recurrence at 2 years is as follows:
• Overall: 42%
• Idiopathic seizure, normal electroencephalogram (EEG): 24%
• Remote symptomatic, epileptiform EEG: 65%
Remote symptomatic means that the seizure is presumably due to a remote brain abnormality or insult. Thus, based on a history, neuroimaging, and an EEG, it may be possible to distinguish between a risk of seizure recurrence that can be as low as 1 in 4 or as high as 2 in 3. There is little doubt that such a distinction may significantly impact the decision to treat. Also, additional risk factors for seizure recurrence have been identified, such as partial (focal onset) seizure, prior acute symptomatic seizure (ie, history of a seizure during an acute neurological disease or insult), the first seizure represents status epilepticus, or the seizure was followed by a Todd’s paralysis. Treatment is often withheld initially in certain forms of epilepsy, such as benign childhood epilepsy with centrotemporal spikes (rolandic epilepsy) or benign occipital epilepsy. Inversely, AED treatment is often initiated in certain conditions associated with rare seizures or no seizures, such as acquired epileptic aphasia (Landau-Kleffner syndrome) or the syndrome of electrical status epilepticus of sleep (ESES).
 SELECTING THE MEDICATION AND STARTING TREATMENT
SELECTING THE MEDICATION AND STARTING TREATMENT
Once the decision to initiate prophylactic treatment has been reached, and before selecting an AED of first choice in monotherapy, every attempt should be made to reach the most accurate diagnosis of the seizure type and, if applicable, of the epilepsy syndrome. The same seizure type may respond to therapy differently, depending on the syndrome in which it occurs. When there is uncertainty about the seizure type or syndrome diagnosis, it is always better to select a drug with a relatively broad spectrum of efficacy. As a general rule, when various drugs have been found to be effective against a given seizure type, it has usually never been demonstrated that one is more effective than the others. Therefore, among the drugs that are considered to be most effective against the patient’s seizure type or syndrome, the drug of choice will be selected predominantly on the basis of its anticipated adverse effect profile in the individual patient, as well as on pharmacokinetic properties, including the possibility of interactions with other medications that the patient may already be taking. In addition to lack of interactions, desirable pharmacokinetic properties include constant bio-availability, a long half-life, low protein binding, and linear kinetics. When considering side effects, it is important to separate the drugs into those that are very unlikely and those that are likely to cause severe, irreversible, or potentially life-threatening side effects. All things being otherwise equal, the latter drugs should not be chosen as first-line therapy. Age or gender of the patient may also be a consideration when the side-effect profile is analyzed. For example, fatal liver failure as a complication of treatment with valproate has been shown to have a much higher incidence in young children (less than 3 years) who took valproate in combination with other AEDs.5 Also, the risk of a severe skin reaction to lamotrigine is higher in children than in adults. The risk of rash with lamotrigine is higher among those who already developed a rash with another AED. Inversely, fatal aplastic anemia related to treatment with felbamate was never reported in anyone less than 18 years old, and hyponatremia with oxcarbazepine is more common in adults and in elderly patients than in children. Also, certain underlying conditions may represent a contraindication for certain drugs, such as valproate in mitochondrial disorders. Gender may also be an issue when the impact of AED side effects is assessed, such as polycystic ovary syndrome with valproate, hirsutism with phenytoin, or teratogenicity with various AEDs. Before prescribing any AED, it is imperative to discuss all common and all potentially serious side effects of the drug with the parents or with the patient. It is also good practice to document what information has been provided. Recently, the FDA alerted the medical community that AEDs in general have been found to be associated with an increased risk of depression and suicidality. Finally, among the adverse effects of AEDs that must be weighed when selecting a drug of choice, one has to take into account the evidence that some drugs can aggravate seizures or cause new seizure types in patients with certain seizure types or epileptic syndromes.6 For instance, it has been shown that carbamazepine, phenytoin, and lamotrigine7,8 can exacerbate myoclonic seizures in patients with juvenile myoclonic epilepsy. It appears that drugs with a broader spectrum are less likely to do so.
After selection of what is considered to be the best drug for the patient, it should be introduced as monotherapy. There is virtually never a good reason to start a patient on more than one AED. An exception may be the temporary addition of a drug with rapid onset of action to a drug that needs to be introduced slowly. Most newer drugs must be introduced gradually, with the goal of achieving the initial target dose after about 2 to 6 weeks. There is only one optimal final dose in a given patient: as much as necessary and as little as possible. The goal of the therapy should always remain the same: no seizures and no side effects. Before switching to another AED or adding a second drug, the dose of the first drug should be increased gradually until either the seizures are controlled, or the patient’s maximal tolerated dose is reached. Combination of two or more drugs should only be considered if there is convincing evidence that the combination works better than either drug alone.
Blood levels of the drugs should only be measured if there is a specific question that might be answered by knowing the blood level. According to recent guidelines, “situations in which AED measurements are most likely to be of benefit include (1) when a person has attained the desired clinical outcome, to establish an individual therapeutic concentration which can be used at subsequent times to assess potential reasons for a change in drug response; (2) as an aid in the diagnosis of clinical toxicity; (3) to assess compliance, particularly in patients with uncontrolled seizures or breakthrough seizures; (4) to guide dosage adjustment in situations associated with increased pharmacokinetic variability (eg, children, the elderly, patients with associated diseases, drug formulation changes); (5) when a potentially important pharmacokinetic change is anticipated (eg, in pregnancy, or when an interacting drug is added or removed); (6) to guide dose adjustments for AEDs with dose-dependent pharmacokinetics, particularly phenytoin.”9 According to common practice, laboratory tests such as complete blood count and transaminases are ordered before introduction of certain drugs and periodically thereafter. This is done only for certain drugs, whereas other drugs generally do not require any routine blood monitoring.
Decision to Discontinue Treatment
It is a widely accepted practice to treat a child with seizures for 2 years after the last seizure. At that point, discontinuation can be discussed with the child and the parents. It is common practice to record an electroencephalogram (EEG) before discontinuing the medications. In certain cases, one may choose to continue the treatment beyond 2 years without seizure. This is based on an assessment of the risk of seizure recurrence if the drugs are stopped. Overall, the risk of seizure recurrence is lower in children (about 20% to 30%) than in adults (about 40% to 50%). In a meta-analysis of 25 studies,10 the risk of relapse was 25% at 1 year and 29% at 2 years. Risk factors associated with higher rates of seizure recurrence include remote symptomatic etiology (ie, preexisting underlying neurological abnormality), seizure onset after the age of 12 years, family history of epilepsy, slowing on the EEG, a history of atypical febrile seizures, and an IQ of less than 50.11 One may also be more hesitant to taper the medication of the patient has a history of status epilepticus. The drug must always be tapered slowly, over a period of about 2 to 3 months. In case of seizure recurrence, the last drug is reintroduced in most instances. Parents and patients often express the concern that the drug may not work again after it has been stopped. This is very uncommon.
CLINICAL CHARACTERISTICS OF INDIVIDUAL DRUGS
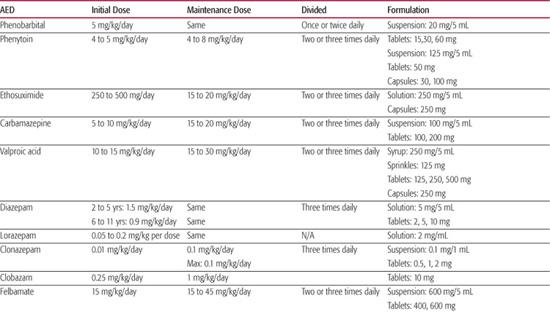
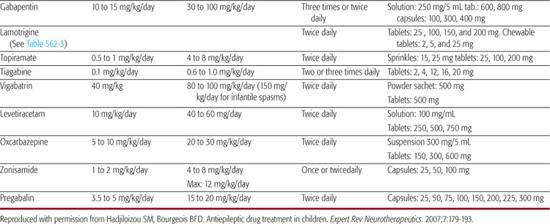
Dosages of antiepileptic drugs (AEDs) are provided in Table 562-1 and their spectrum of efficacy, indications and main adverse effects are shown in Table 562-2. Specific dosage recommendations for lamotrigine are provided in Table 562-3. Further discussion of each antiepileptic drug, including their pharmacokinetics, common drug interactions is provided in electronic text. 
DRUGS OF FIRST CHOICE AND TREATMENT PARADIGMS FOR SPECIFIC SEIZURES AND SYNDROMES
Table 562-4 represents an attempt to rank antiepileptic drugs (AEDs) as first and second choices for various seizure types and epilepsy syndromes occurring in the pediatric age group. Because scientific evidence alone cannot be used to establish such rankings, the choices have to be based on a combination of published evidence of efficacy, side effect and safety considerations, published and personal uncontrolled observations, and comfort factor. Accordingly, such rankings may appear somewhat arbitrary and are open to debate and to change over time (Table 562-4).
 PARTIAL SEIZURES
PARTIAL SEIZURES
Overall, most AEDs have documented efficacy against partial seizures, and none could be shown to be more effective than any other. Therefore, choices should be based mainly on side effect profile and overall accumulated experience with the different AEDs. Accordingly, carbamazepine (CBZ), oxcarbazepine (OXC), and levetiracetam (LEV) could be considered as drugs of first choice.
 GENERALIZED TONIC-CLONIC SEIZURES (GTCS)
GENERALIZED TONIC-CLONIC SEIZURES (GTCS)
The choice of medication for GTCS may depend on whether the patient is experiencing a nonfocal GTCS in the context of idiopathic generalized epilepsy. If this is the case, the drugs of choice would be valproic acid (VPA), lamotrigine (LTG), levetiracetam (LEV), or topiramate (TPM) because of the available evidence. Carbamazepine (CBZ), phenytoin (PHT), or zonisamide (ZNS), which are potentially effective, could be considered as further choices, but CBZ and PHT should be avoided if an idiopathic generalized epilepsy is suspected.
Table 562-1. Antiepileptic Drug (AED) Formulations and Dosages in Children
 ABSENCE EPILEPSIES: CHILDHOOD AND JUVENILE ABSENCE EPILEPSY (CAE AND JAE)
ABSENCE EPILEPSIES: CHILDHOOD AND JUVENILE ABSENCE EPILEPSY (CAE AND JAE)
Children with absence seizures may be divided into 2 age categories: those under the age of 10 years and those above the age of 10 years. There are at least 3 reasons for this: (1) concomitant GTCS are more likely to occur after the age of 10 years in patients diagnosed with CAE, and are common in JAE; (2) the incidence of VPA-induced fatal hepatotoxicity is highest in infants and young children, especially in combination therapy; and (3) the incidence of severe hypersensitivity reaction associated with LTG also appears to be inversely age related. Consequently, Ethosuximide (ESM) may still represent the drug of first choice in patients younger than 10 years old who have only absence seizures, as is usually the case in CAE. In any child older than 10 years with absence seizures, when an AED needs to be introduced or changed, the choice would be valproic acid (VPA). It is quite safe in monotherapy at this age and highly effective against GTCS, whereas ESM offers no such protection. LTG, which is also effective against absence seizures and GTCS, is quite safe in this age group and is a valuable alternative to VPA, especially in adolescent girls. 
 JUVENILE MYOCLONIC EPILEPSY (JME)
JUVENILE MYOCLONIC EPILEPSY (JME)
Valproic acid (VPA) remains a drug of choice for JME. However, the side-effect profile of VPA is a concern, especially in women of childbearing age (weight gain, teratogenicity, the possibility of polycystic ovary syndrome, and cognitive impairment in a child exposed to VPA in utero). For a potentially lifelong condition such as JME, an alternative drug would be desirable, such as LTG that has a much lower incidence of severe idiosyncratic reactions in adults than in children, and has few other side effects. Lamotrigine (LTG) is quite effective against GTCS, but it may aggravate myoclonic seizures. There is growing evidence that levetiracetam (LEV) is effective in all seizure types of IGE and is another promising alternative to VPA. Clonazepam is very effective against the myoclonic seizures but less effective against the GTCS. TPM has been shown to be effective against the GTCS in JME,19 but its role against myoclonic seizures remains to be established.
Table 562-2. Main Indications and Side Effects of Antiepileptic Drugs (AEDs)



