Environmental Pediatrics
Ruth A. Etzel
It is only in the last 50 years that diseases linked to environmental contamination have been recognized in pediatrics. Most environmental exposures now understood to be harmful to children were initially identified as a result of acute epidemics of illness affecting groups of people. For example, polluted outdoor air was not well understood to be unhealthy until the London Smog of 1952.1 An estimated 4000 people died from exposures to the very heavy air pollution; deaths were unusually high among the very young and the elderly. This led to the development of the first laws to regulate air pollution. Mercury was not understood to be harmful until an epidemic of cerebral palsy occurred among infants living near Minamata Bay, Japan, in the 1950s (Minamata disease).2 Between 1959 and 1972 in Iraq, seed grain treated with a mercury fungicide was accidentally eaten by humans instead of being planted in the fields, and thousands of Iraqi people developed mercury poisoning.3,4
One of the most important themes that emerged from these acute epidemics of environmental diseases was that, unlike some other specialty areas of pediatrics, in environmental pediatrics, treatment of the individual patient is not sufficient; it is critical to identify and eliminate the sources of exposure so that disease does not recur in the known patients and so that additional cases do not occur.
DIAGNOSING ENVIRONMENT-RELATED ILLNESS
Most signs and symptoms of illnesses linked to environmental contaminants are nonspecific and may occur only in association with fairly high-level exposures. The signs and symptoms of high-level, acute poisoning are relatively well-characterized for most chemicals. In contrast, the effects of low-level, chronic, and mixed exposures are poorly studied, particularly in infants and children. Establishing the environmental cause of a given illness is further complicated by the similarity of pollutant effects to those of other diseases. For example, a respiratory illness from an environmental pollutant may closely resemble an allergy or a respiratory infection. Diagnosis of an environmental disease requires a high index of suspicion and a thorough environmental history. While clinicians cannot assess the causal association between pollutant exposures and health effects, they should consider environmental causes of disease when children’s illnesses do not follow usual expected patterns.
A comprehensive intake of a new patient and ongoing anticipatory guidance should include an assessment of possible environmental risks to the child in the home environment. This includes discussion of the source of drinking water, potential access or exposure to toxins in the home environment or parental workplace and assuring that appropriate environmental monitoring is performed in the home, including fire and carbon monoxide detectors. Anticipatory guidance differs by the age of the child because environmental risks differ by age. When the baby is 2 months old, the clinician should ask about nitrate in drinking water, smoking, and water damage and mold growth in the home. At 6 months, clinicians should ask about lead in the home. When the child reaches school age, the pediatrician should ask questions about radon, mercury, carbon monoxide, ultraviolet light, and pesticides. Families should be advised to prevent excessive exposure to contaminants and their possible adverse effects. Commonly encountered environmental toxins and pollutants are described individually in this chapter. Discussions of specific toxins and management of exposures are also found in Chapter 120.
CARBON MONOXIDE
Carbon monoxide (CO) is an odorless gas produced by incomplete combustion of carbon-containing material. Common sources of exposure include house fires, automobile exhaust, and improperly ventilated water heaters, stoves, furnaces, fireplaces, and space heaters. Inhalation of CO is the leading cause of poisoning death in the United States. The diagnosis of carbon monoxide poisoning is readily suspected in the patient found unconscious in a house fire or running automobile. However, the diagnosis is difficult when the patient presents with non-specific symptoms after an unrecognized exposure. Mild to moderate exposures present with malaise, nausea, vomiting, dyspnea, headache, dizziness, and confusion. Such patients are often misdiagnosed with influenza or gastroenteritis. Severe poisoning is characterized by syncope, seizures, coma, cardiorespiratory depression, and death. Diagnosis and treatment of carbon monoxide exposure is discussed in detail in Chapter 120.
LEAD
The major sources of lead exposure among children in the United States is ingestion of lead-containing paint chips or lead-contaminated dust or dirt by hand-to-mouth activity. Although lead-based paint was banned in 1978 for use in homes, as many as three quarters of dwellings built before 1980 have lead-containing paint on their interior surfaces. Lead paint on interior and exterior window components becomes abraded into dust by repetitive opening and closing of the window. Lead poisoning also may result from use of certain herbal remedies, traditional cosmetics, imported spices, and ceramic cooking utensils. Lead poisoning has been caused by drinking water contaminated from lead pipes used in some older municipalities, and solder in home plumbing. Inhalation of lead-containing air may occur in children living in the vicinity of lead smelting and automobile battery plants and from clothing brought into the home by family members working in lead-related industries. Fishing weights, stained glass or ceramics may also lead to exposure. Gasoline containing tetraethyl lead, used to prevent engine “knocking,” was extensively used in the United States until the early 1980s and contributed to an increased concentration of lead in the soil.15
Lead serves no physiologic function. Ingested material containing lead is solubilized by gastric hydrochloric acid and then absorbed primarily in the upper gastrointestinal tract. Although adults absorb about 10% of ingested lead, children absorb as much as 50%. Absorption may be increased further by deficiencies of iron and other trace metals, malnutrition, and increased fat in the diet. About 70% to 90% of absorbed lead is deposited in the bones and about 5% is present in the red blood cells and their precursors in the bone marrow. Lead is very slowly excreted from the body, primarily in the urine, and its biological half-life has been estimated to be more than 15 years. The toxic effects of lead are primarily related to its binding to sulfhydryl ligands, leading to inhibition of a large number of enzymes. Lead toxicity is a function of the level of lead in the blood and tissues as well as the duration of exposure. In the red blood cell precursors, lead interferes with several steps in the heme synthetic pathway, which leads to an increased level of heme precursors, free erythrocyte protoporphyrin, and zinc protoporphyrin, in the mature red blood cells. At higher levels, lead reduces iron utilization, resulting in anemia.
In the immature, developing brain, even moderate elevations of lead are associated with neurobehavioral abnormalities and decreased intelligence quotients, which appear to be permanent. Lead can cause neuronal demyelinization, decreased numbers of neurons, decreased neuronal growth, interference with neuronal transmission, and sensorineural hearing loss that may result in significant delays in the acquisition of language and difficulty in auditory processing. At very high levels, lead can cause encephalopathy and cerebral edema, resulting in death or severe neurologic damage in survivors. Peripheral neuropathy and nephropathy that occur in lead-poisoned adults are unusual in children.
The magnitude of body lead is indicated by the blood lead level (BLL), and laboratory assessment is based primarily on blood levels determined by atomic absorption analysis.  Recommendations regarding screening for lead exposure are reviewed in Chapter 12.
Recommendations regarding screening for lead exposure are reviewed in Chapter 12.
In 1991, the Centers for Disease Control and Prevention defined 10 μg/dL as the BLL that should prompt public health actions. This level does not, however, define a threshold for the harmful effects of lead. The physical and mental development of children can be affected at BLLs of less than 10 μg/dL.16,17
Symptoms that may be associated with lead poisoning are relatively nonspecific and include gastrointestinal complaints such as anorexia, constipation, abdominal pain, and vomiting. Signs and symptoms suggestive of central nervous system involvement include irritability, lethargy, changes in sleep pattern, and alterations in behavior and coordination. Seizures, hypertension, coma, and signs of increased cranial pressure are indicative of lead encephalopathy, which is usually associated with blood levels higher than 70 μg/dL.
In all exposed children, a health department inspection should be conducted to ascertain possible sources of lead in the home. Until this is possible, the child should be relocated to a known lead-free environment. If home therapy is being considered, a health department inspection should be done prior to starting treatment. A randomized controlled trial indicated that chelation therapy for children with BLLs between 25 and 45 μg/dL did not have a beneficial effect on chronic neuropsychological abnormalities; therefore, chelation therapy is usually reserved for children with a BLL higher than 45 μg/dL.18 However, medical evaluation, repeated testing, nutritional intervention to increase iron and calcium intake and decrease fat consumption, treatment of iron deficiency anemia, and investigation of the source of lead exposure are indicated. In the absence of clinical symptoms suggesting encephalopathy, children with lead levels of 45 to 69 μg/dL further management includes bowel decontamination if an abdominal radiograph shows possible lead particulate matter in the gastrointestinal tract, and chelation therapy. Chelation therapy can be started with 2,3-dimercaptosuccinic acid (succimer; DMSA) at 30 mg/kg per day for 5 days, followed by 20 mg/kg per day for 14 days. An alternate regimen is to use CaNa2EDTA as inpatient therapy at 25 mg/kg per day for 5 days. If there are unacceptable reactions to DMSA and CaNa2EDTA treatment with penicillamine can be considered at doses of 20 to 30 mg/kg per day for a duration of 4 to 12 weeks. The toxicity of penicillamine relegates it to use as a third line agent. In all of these children, follow-up neurodevelopmental monitoring should also be assured.
In children with a lead level over 70 μg/dL or symptoms suggesting encephalopathy require inpatient chelation therapy with parenteral agents. If encephalopathy is present intensive care treatment including intracranial pressure monitoring, and therapy for intracranial hypertension is mandatory, in addition to chelation therapy. Therapy is initiated with intramuscular dimercaprol (BAL) at 25 mg/kg per day divided into six doses. A second dose of BAL is given 4 hours after the initial dose, followed immediately by intravenous CaNa2edta AT 50 mg/kg per day as a single dose infused during several hours or as a continuous infusion. Adequate hydration must be ensured to maintain renal excretion. Therapy is continued for at least 72 hours, or until encephalopathy resolves. The American Academy of Pediatrics treatment guidelines for lead exposure are available at: http://aappolicy.aappublications.org/cgi/reprint/pediatrics;96/1/155.pdf. Another helpful resource is available at: A useful summary of the approach treatment of lead exposure is provided at http://www.cdc.gov/nceh/lead/casemanagement/caseManage_chap3.htm.
MERCURY
Mercury has been used for more than 3000 years in medicine and industry. Mercury occurs in three forms: the metallic element (quicksilver or elemental mercury), inorganic salts (mercurous or mercuric salts), and organic compounds (methylmercury, ethylmercury, and phenylmercury).
Elemental mercury has been used in thermometers, sphygmomanometers, and thermostat switches. Dental amalgams contain mercury, silver, and other metals. Fluorescent light bulbs (usually 2–4 ft tubes) and disk (“button”) batteries also contain mercury. Elemental mercury also is used in some folk remedies.19,20
Elemental mercury is a liquid at room temperature and readily volatilizes to a colorless and odorless vapor. When inhaled, it easily passes through pulmonary alveolar membranes and distributes into red blood cells and the central nervous system. Less than 0.1% is absorbed after ingestion, and only minimal absorption occurs through the skin.5
Inhalation of high concentrations of mercury vapor can cause a fatal acute necrotizing bronchitis and pneumonitis.21 Fatalities have resulted from heating elemental mercury in inadequately ventilated areas.22
Children may present with rash, vomiting, muscle pain, and tachycardia. Clinicians may miss the diagnosis unless they ask the child about mercury exposures.
Long-term exposure to mercury vapor primarily affects the central nervous system. Early signs include insomnia, forgetfulness, loss of appetite, and mild tremor. These symptoms may be misdiagnosed as a psychiatric illness. Continued exposure results in progressive tremor and erethism, characterized by red palms, emotional lability, and memory impairment. Salivation, excessive sweating, and hemoconcentration may follow. 
 INORGANIC MERCURY
INORGANIC MERCURY
Acrodynia, or childhood mercury poisoning, was frequently reported in the 1940s among infants exposed to calomel teething powders containing mercurous chloride.24,25 Acrodynia has developed in infants exposed to phenylmercury used as a fungicidal diaper rinse26 and in children exposed to phenylmercuric acetate from interior latex paint.27
Inorganic mercury exposure is measured by urinary mercury determination, preferably using a 24-hour urine collection. 
Mercury accumulates in blood and in central nervous system and renal tissues and is very slowly eliminated. Chelating agents have been used to enhance mercury elimination, but whether chelation reduces toxic effects or speeds recovery in persons who have been poisoned is unclear. 
 ORGANIC MERCURY
ORGANIC MERCURY
Unlike inorganic mercury, organic mercury compounds are lipid soluble and essentially 100% absorbed after ingestion. Ethylmercury is found in thimerosal, an antiseptic and preservative previously used for vaccines and other drug therapies. Currently, all routinely recommended vaccines manufactured for administration to infants in the United States are available in thimerosal-free formulation.
Organic mercury toxicity affects the central nervous system. Signs progress from paresthesias to ataxia, followed by generalized weakness, visual and hearing impairment, tremor and muscle spasticity, and then coma and death. 
Blood mercury levels rarely exceed more than 1.5 μg/dL in the unexposed population, and a blood concentration of 5 μg/dL or greater is considered the threshold for symptoms of toxicity. Methylmercury also distributes into growing hair, thus providing a noninvasive means to estimate body burden and blood concentration over time. In the general population, the mercury level in hair is usually 1 ppm or less.39-42 There is no chelation agent approved by the US Food and Drug Administration (FDA) that is effective for methylmercury poisoning, although succimer has been used for the treatment of severe organic mercury poisoning.
Children who have had mercury poisoning should undergo periodic follow-up neurologic examinations by a pediatrician.
Guidelines for maximum exposure to mercury have been established by the Environmental Protection Agency at 0.1 μg/kg/d, by the FDA at 0.4 μg/kg/d, and by the Agency for Toxic Substances and Disease Registry at 0.3 μg/kg/d. The FDA and most states advise that pregnant women, young children, and women of childbearing age should not eat fish with mercury levels greater than 0.5 ppm. Most state health agencies advise limiting intake of freshwater fish having greater than 0.2 ppm of mercury, and the FDA advises that people at higher risk eat no more than 12 oz of fish a week, on average. Other states simply recommend that women and children not eat any large predator fish that tend to concentrate mercury.43 The levels of mercury in commercial fish and shellfish are reported at: http://www.fda.gov/Food/FoodSafety/Product-SpecificInformation/Seafood/FoodbornePathogensContaminants/Methylmercury/ucm115644.htm. In the United States, state specific recommendations for consumption of sport fish are available on the Environmental Protection Agency Web site: http://www.epa.gov/OST/fish/. 
NITRATES AND NITRITES IN WATER
High nitrate levels in water can have adverse health effects. Nitrate and other nutrients have been linked to blue-green algal blooms, which can produce toxic bacteria.45,46 Methemoglobinemia in infants may be caused by ingestion of water contaminated with nitrate, that is converted to nitrite by intestinal bacteria, which then oxidizes fetal hemoglobin to methemoglobin. Infants younger than 4 months of age who are fed formula reconstituted with nitrate-containing well water are at the greatest risk.
Evaluation and treatment of methemoglobinemia is discussed in Chapter 120. It is critical to identify and eliminate the exposure source prior to discharge. Reconstitution of formula with well-water is generally not recommended and, if used, well-water should be tested for safety.
N-nitroso compounds are some of the strongest known carcinogens and induce cancer in a variety of organs in more than 40 animal species, including higher primates. Exposures to nitrate in drinking water may be associated with increased risks of non-Hodgkin lymphoma, gastric cancer, bladder cancer, hyperthyroidism, and insulin dependent diabetes.47-53
PESTICIDES
Pesticides are widely available consumer products. Many aerosol products marketed for use in the home, sometimes called “over-the-counter bug bombs,” contain organophosphate and carbamate insecticides. Insecticides for garden and agricultural uses have a higher potential for toxicity because they are intrinsically more toxic and more concentrated. Acute organophosphate and carbamate toxicity diagnosis and management is discussed in Chapter 120.
Long-term effects of low-level exposures to pesticides have been documented. Organophosphate exposure has been linked to neurodevelopmental delays.54 Epidemiologic studies demonstrate associations between pesticide exposures and certain childhood cancers, including leukemia, all brain cancers, and non-Hodgkin lymphoma. A comprehensive review of studies showing an association between pesticides and childhood leukemia revealed higher risks among children whose parents were exposed to pesticides at work or who used pesticides in the home or garden. Several studies have linked home use of pesticides with childhood brain tumors. Using sprays or foggers to dispense flea or tick treatments, flea collars, home pesticide bombs, fumigation for termites, and pest strips were associated with having brain tumors. Studies in agricultural workers linked chronic exposures to chlorophenoxy herbicides with non-Hodgkin lymphoma. Pediatricians should ask questions about exposures to pesticides at home and suggest adoption of integrated pest management programs.
POLYCHLORINATED BIPHENYLS, DIBENZOFURANS, AND DIBENZODIOXINS
Polychlorinated biphenyls (PCBs) are clear, non-volatile, hydrophobic oils that resist metabolism and persist in the environment. They were used primarily in the electrical industry as insulators until the late 1970s, when they were banned in the United States and northern Europe. There are still detectable levels of PCBs in human tissue and human milk. Polychlorinated dibenzofurans (PCDFs) are partially oxidated PCBs.
The source of exposure for most people to all of these compounds is contaminated food. Because the chemicals are not well metabolized or excreted, even very small daily doses accumulate to measurable amounts over years. The most concentrated source is sport fish from contaminated waters because the residues bioconcentrate, and fish usually is the food at the highest trophic level consumed by humans. The major dietary source for young children is human milk.
Chronic exposure to commonly encountered levels of PCBs is associated with lower developmental and intelligence test scores, including lower psychomotor scores, defects in short-term memory, and lowered intelligence quotients.55-60 Acute exposure has been documented twice in Asia, and affected children are found to have persistent behavioral abnormalities and cognitive impairment.61
Although many laboratories can measure PCBs, there are no agreed-on methods, quality assurance programs, or for diagnostic or therapeutic use. No regimen is known to lower body burden of these compounds.
Fish advisories for Lake Michigan recommend that individuals not eat more than one meal per month of salmon (with an average of 0.7 ppm PCBs), for a maximum of 12 meals per year. Women and children are advised to wait a month before eating another meal of Lake Michigan salmon to prevent PCBs from building up in the body.
PCB exposures can be reduced by cleaning (eg, fat removal) and cooking methods.
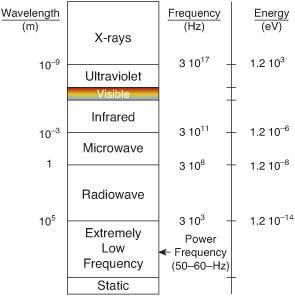
IONIZING RADIATION
Types of radiation are shown in Figure 17-1. On average, the annual effective dose equivalent of ionizing radiation to a person in the United States is 0.006 Sv (0.6 rem), 37% of which is from radon, 13% from other natural sources, 24% from computerized tomography, 12% from nuclear medicine, 7% from interventional fluoros-copy, 5% from medical x-rays, and 2% from other man-made sources (see Fig. 17-2). 
Acute effects of overexposure include acute radiation sickness (nausea, vomiting, diarrhea, declining white blood cell count, and thrombocytopenia), epilation (loss of hair), and death. Delayed effects largely are due to mutagenesis, teratogenesis, and carcinogenesis. 
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


