Environmental Causes of Birth Defects
Elizabeth Balken, Marsha Leen-Mitchell, Lynn P. Martinez, and Julia A. Robertson
The definition of a teratogenic agent (an exposure, external to the fetal genome, that induces structural or functional alterations during prenatal development) suggests that the clinicians’ first encounter may be well after the exposure has occurred. Knowledge of the principles of teratology can direct diagnostic and treatment approaches to a child with dysfunction or dysmorphology of unknown etiology and can be instrumental in guiding initial evaluations as well as physician–patient–parent interactions.
FACTORS USED TO DETERMINE TERATOGENIC RISK
In weighing the possibility that a teratogen is responsible for a child’s condition or defect, one of the most important factors to consider is background risk. Every pregnancy carries an approximate 3% risk that the fetus will be affected with a major, life-affecting defect. Although this level of risk can be increased by maternal teratogenic exposure, it cannot be decreased. A common misconception is that a majority of birth defects are the result of teratogenic exposure. However, teratogens cause only about 3% of the clinically significant congenital structural defects in humans. Because no teratogen affects all exposed fetuses, there is probably an interaction between genes and environment necessary to create teratogenicity.
To determine risk, the following factors must be assessed:
Timing of exposure
Dose of the agent
Route
Duration of exposure
Confounders
Species susceptibility
Clinical sonsistency
 TIMING OF EXPOSURE
TIMING OF EXPOSURE
The time frame at which an exposure occurs during the pregnancy is, arguably, the most important factor to consider when determining teratogenicity. Contemporary understanding of embryology and embryopathy guides this principle. For example, teratogenic exposure between days 15 and 28 after fertilization can be the cause of a neural tube defect, because during this time neural tube closure occurs in the embryo. Parallels can be drawn between teratogenic exposure and development of virtually any other major organ or system. Thus, knowing that the mother was exposed during a critical time for induction of the child’s anomaly is vital when evaluating a child for possible teratogenic effects.
 DOSE OF THE AGENT
DOSE OF THE AGENT
Fortunately, threshold levels have been estimated for most known human teratogens. For example, the dosage of methotrexate required for therapeutic treatment of rheumatoid arthritis and psoriasis is considerably below that which could induce fetal defects.
 ROUTE OF EXPOSURE
ROUTE OF EXPOSURE
Most agents are transmitted to the fetus indirectly, through maternal blood. Therefore, in order for the fetus to be affected, an agent must have access to maternal blood. The amount of fetal exposure to compounds concentrated in maternal blood is, therefore, dependent on maternal blood levels. Isotretinoin, a known teratogen, is directly absorbed into the maternal bloodstream, whereas tretinoin, a topical vitamin A congener, has minimal maternal systemic absorption and is not associated with an increased risk for birth defects.
 DURATION OF EXPOSURE
DURATION OF EXPOSURE
Since functional as well as structural development of the fetus can be affected by some teratogens, the duration of an exposure can have a significant impact. For example, smoking more than 10 cigarettes each day can affect the growth of the fetus if the smoking continues beyond the twelfth week of gestation. Alcohol can affect functional brain development, and thus heavy consumption of alcohol that continues beyond the first trimester is associated with developmental and learning disorders.
 CONFOUNDING VARIABLES
CONFOUNDING VARIABLES
Confirmation of a specific drug, chemical, infection, or other exposure as cause for an adverse outcome can be complicated by the fact that multiple exposures often take place concurrently in the same pregnancy. As an example, early investigations into caffeine led researchers to hypothesize that its consumption during pregnancy resulted in low-birth-weight infants. Later it was discovered that immoderate coffee drinkers are also more likely to be immoderate tobacco users; smoking was thus the confounding variable in lower birth weight.
 SPECIES SPECIFICITY
SPECIES SPECIFICITY
Studies of pregnancy outcomes in animals are not always predictive of human outcomes, because teratogenic agents tend to demonstrate species-specific effects. Genetic variability among species produces differences in drug absorption, distribution, and metabolism. Extrapolating from animal data to humans is problematic, because pharmacokinetic profiles vary depending on the drug and species. For example, the limb deficiency observed in children with thalidomide exposure is not seen in the usual animal studies. Other differences may be credited to animals’ ability to carry multiple fetuses and variations in placental development and function. Although animal models are useful in detecting the mechanisms by which known teratogens exert their influence, such studies are not beneficial in determining which agents are teratogenic in humans.
 CLINICAL CONSISTENCY
CLINICAL CONSISTENCY
Most of the identified human teratogens show patterns of abnormalities presenting as syndromes rather than isolated or nonspecific single defects. Although statistically rare, these events have been the hallmark by which teratogens have been “discovered.” Two examples can illustrate this point.
Within three years of release of isotretinoin, three cases of isotretinoin embryopathy characterized by isolated anotia, microtia, and conotruncal heart defects were reported. All three infants had a combination of these anomalies that is rarely seen, heightening the statistical probability that there was a teratogenic agent involved. Based on just three reports, isotretinoin was suspected as a human teratogen; this was later established by epidemiologic and clinical investigations.
Case reports of malformations among children exposed to Bendectin, a combination of pyridoxine (vitamin B6), doxylamine (Unisom), and dicyclomine (Bentyl) in utero were reported, but no pattern of defects could be detected. Epidemiologic analyses found the rate of malformations in patients exposed to the drug was no higher than the background risk of malformations. This demonstrated the lack of teratogenicity of the product.
In summary, when assessing causation, the degree of certainty of etiology depends on the rarity of the exposure and the distinctiveness of the outcome: the rare exposure, rare outcome, astute clinician model. Table 183.1 provides a complete list of known human teratogens. What follows is a discussion of selected teratogens.
 DRUGS
DRUGS
Few pregnancies progress to term without the use of at least one medication, whether prescription or nonprescription drugs, herbal remedies, or nutritional supplements. An average of three to four medications are used during the course of a pregnancy, with analgesics being the most commonly reported. About 30% of women are exposed to a prescription medication during the first trimester of pregnancy. Other common categories include cough and cold products, antacids, antihistamines, antiemetics, psychotropics or sedatives, and antibiotics.
Valproic Acid
Valproic acid (Depakote) carries a 1% risk for a neural tube defect (spina bifida) when fetal exposure occurs between 15 and 29 days after conception. Other fetal effects (craniofacial changes, preaxial defects, and hypospadias) have also been described, but the level of risk for these effects has not been established.
Methotrexate/Aminopterin
Methotrexate can have a teratogenic effect when taken between 8 and 11 weeks after the last menstrual period at doses higher than 10 mg per week. The level of fetal risk after exposure to methotrexate is not known. Craniosynostosis and craniofacial abnormalities (wide-spaced eyes, broad nose, small chin, and flattened facies), and limb defects (absent toes, webbed fingers, or shortened limbs) have been reported. Based on cases in which aminopterin was used as an abortifacient in high doses (12 mg or more per week), there is an increased risk for spontaneous abortion, low birth weight, craniofacial abnormalities, limb abnormalities, craniosynostosis, and possibly neural tube defects (spina bifida or anencephaly). The level of risk for birth defects associated with aminopterin use in the first trimester of pregnancy is unknown.
Table 183-1. List of Known Human Teratogens
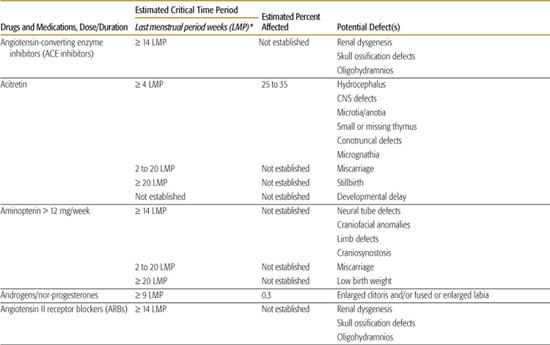
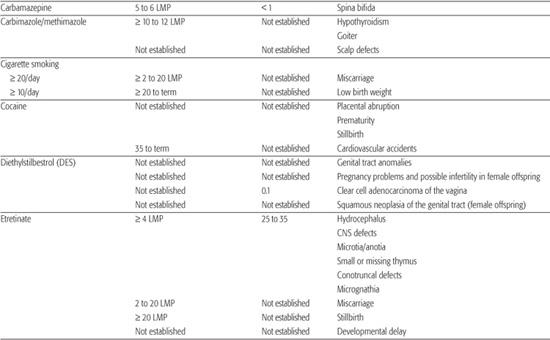
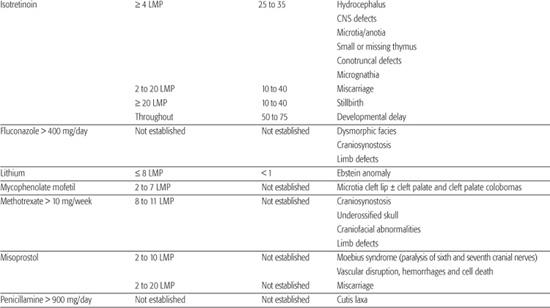
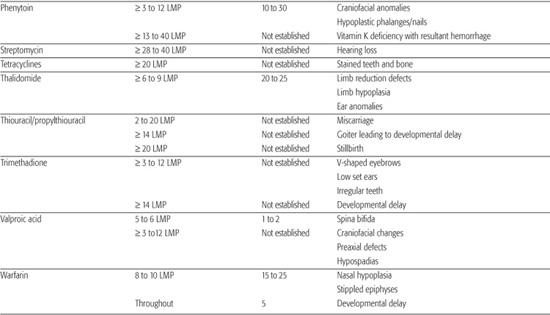
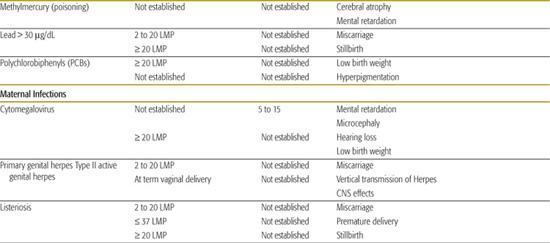
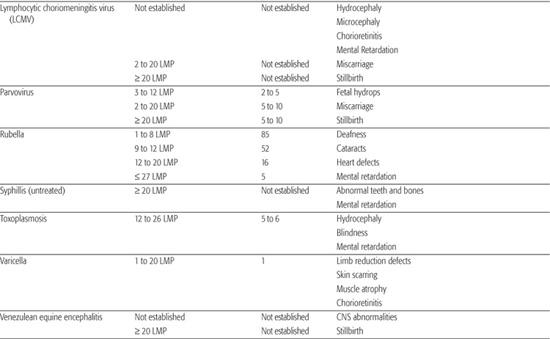
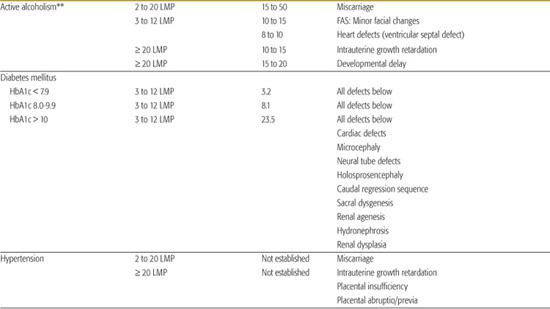
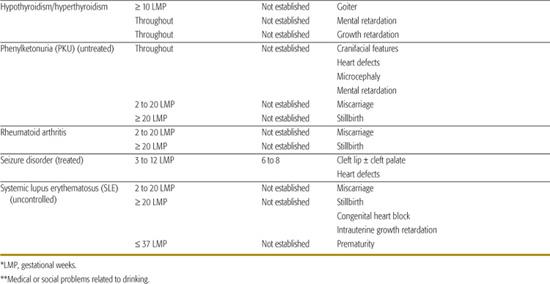
Thalidomide
Thalidomide was the first drug identified as a human teratogen. When exposure to the drug occurs during days 34 to 50 of gestation (3–6 LMP weeks), there is a risk of at least 20% for limb reduction defects (missing segments of the arms and/or legs) and ear malformations, including deafness. Because of its effectiveness in treating some peripheral neuropathies associated with Hansen disease, thalidomide was approved for marketing in the United States in the summer of 1998. In an effort to avoid additional cases of thalidomide embryopathy in children, the drug’s parent company has established an extensive physician and pharmacy registration process called the System for Thalidomide Education and Prescribing Safety (S.T.E.P.S.) (http://www.thalomid.com/steps_program.aspx).
 MATERNAL DISORDERS
MATERNAL DISORDERS
In most cases with maternal illnesses, risk to the fetus increases with the severity of maternal disease and inadequate treatment, emphasizing the necessity of rigorous medical oversight.
Alcoholism
It is not known if there is a safe amount of alcohol that can be consumed during any trimester of pregnancy. However, it is unlikely that a single drink (12 ounces of beer/5 ounces of wine/1.5 ounces of hard liquor) during pregnancy would affect a fetus or cause fetal alcohol spectrum disorders (FASD).
Binging is defined as drinking to the point of heavy intoxication (being drunk), usually drinking more than 5 drinks (beer, wine, or hard liquor) on any occasion. When a pregnant woman binges more than 3 times in early pregnancy, there is a 15% to 50% risk of miscarriage, a 10% to 15% risk of minor changes in the face and an 8% to 10% risk of a minor heart defect in the fetus. Binge drinking that continues beyond the fifth month of pregnancy is associated with an increased risk for growth delay and a risk of learning disabilities and behavior problems in the child. Women who drink heavily (more than 3 to 4 drinks a day) or binge weekly have a 15% to 50% risk of miscarriage; a 10% to 15% chance of having a child with minor facial defects, growth delay, and a 5% to 25% risk of having a child with FASD.
The fetuses of alcoholic women (or women who experience health problems or social problems related to their drinking) may be at risk even if maternal alcohol consumption is less than the amounts described.
Diagnosing FASD is complicated and should only be done after ruling out other conditions that present with similar effects. For instance, genetic conditions such as Williams syndrome, Cornelia de Lange syndrome, and velocardiofacial syndrome (VCFS) all have similar facial and growth characteristics.
Diabetes Mellitus Types I and II
Maternal diabetes mellitus is the most common cause of birth defects. Mothers with insulin-dependent diabetes have an increased risk for having a child with a congenital malformation. The level of risk is dependant on the mother’s hemoglobin A1c level (HbA1c) (Table 183.2).
The pattern of defects observed with the diabetic embryopathy includes defects of the heart (transposition of the great arteries, pulmonary stenosis, dextrocardia, patent ductus arteriosus), central nervous system (hydrocephaly, microcephaly, holoprosencephaly, neural tube defects), skeleton (sacral dysgenesis, caudal deficiency), and kidneys (renal agenesis, hydronephrosis, multicystic displastic kidneys). Improved control of glucose levels prior to conception decreases the risk substantially and underscores the importance of preconceptional counseling.
 INFECTIONS
INFECTIONS
Primary infections of childhood diseases (eg, human parvovirus B19 and varicella) are of concern in pregnancy. Fetal effects occur only when the pregnant woman acquires the active infection. Even then, only a small percentage of fetuses will contract the disease and have an adverse outcome.
Human Parvovirus B19/Fifth Disease
There have been no confirmed reports of congenital anomalies related to maternal parvovirus infection. Hydrops occurs in 10% of fetuses whose mothers contract this infection during pregnancy. The critical time for fetal effects is between 12 and 26 LMP weeks.
Varicella
When a pregnant woman contracts varicella (chickenpox) during the first trimester, the risk of fetal effects is approximately 1%. If the infection occurs prior to or during limb bud formation, limb reduction defects can result. Other effects of varicella include chorioretinitis, scarring of the skin with muscle atrophy, and a possibility of developmental delay, which is less well established than the eye, skin, and limb effects (see Chapter 314).
 CHEMICALS
CHEMICALS
Given the growing number of chemical agents being developed or sold for both home and industrial environments, pregnant women are increasingly vulnerable to chemical exposures. Although many such compounds do not appear to be teratogenic to humans, limited human data exist to develop risk assessment. In most cases, however, maternal poisoning is necessary for a teratogenic effect to occur. Some selected chemical teratogens are described below.
Methylmercury
Methylmercury is an organic compound that can accumulate in animals (eg, fish) that are subsequently consumed by humans. When a pregnant woman develops symptoms of methylmercury poisoning, there is a concern for prenatal encephalopathy at any stage of the pregnancy. Recent reports of high levels of methylmercury in fish have created confusing messages regarding the safety of fish consumption. Nearly all fish contain trace amounts of methylmercury. Federal agencies recommend limiting fish to 6 ounces weekly for women who are pregnant or lactating. Pregnant or breast-feeding women should avoid eating large fish (shark, swordfish, king mackerel, and tilefish) because they live a long time, feed on smaller fish, and have the highest levels of methlymercury. For the current recommendations on fish consumption see the Federal Drug Administration Web site at http://www.fda.gov/fdac/reprints/mercury.html.
Table 183-2. Risk of Congenital Malformations in Mothers with Diabetes Mellitus Related to Hemoglobin A1c Levels (Percent)

Solvents
Studies of solvent exposure in an occupational setting have shown pregnancy loss when mothers are exposed to high does for long periods and have symptoms of toxicity (lightheadedness and headaches). In the absence of these signs of toxicity, no adverse fetal effects have been confirmed. The fetal effects of solvent abuse are discussed in the following section.
 SUBSTANCES OF ABUSE
SUBSTANCES OF ABUSE
With the exception of alcohol and possibly cocaine and solvents, no substance of abuse has been conclusively associated with an increased risk of birth defects. However, reversible toxicity and/or withdrawal symptoms may occur in newborns whose mothers abuse certain drugs throughout the pregnancy or in large doses near the time of delivery. Substance abuse throughout pregnancy has been associated with an increased risk for intrauterine growth retardation, prematurity, and low birth weight regardless of the particular substance abused. Also, with needle use, an increased risk for transmission of pathogens such as human immunodeficiency virus (HIV) or hepatitis B, C, and D can cause adverse health effects for both the mother and infant.
Cigarette Smoking
Investigative evidence shows a greater risk of low birth weight commensurate with the number of cigarettes smoked during pregnancy. Evidence also suggests that heavy maternal smoking (more than 10 cigarettes/day) is associated with an increased risk for miscarriage, premature delivery, and stillbirth.
Cocaine
With cocaine use during pregnancy, an increased risk for abruptio placentae, which can result in a miscarriage, stillbirth, or premature delivery, occurs. Used near delivery, cocaine can also be associated with an increased risk for intracranial hemorrhage. Infants whose mothers use cocaine continuously throughout pregnancy or in large amounts near the time of delivery may be at an increased risk for irritability, tremulousness, and muscle rigidity, which usually develop several days after birth, resolve quickly, and seem to have no long-term effects on the infant or child.
Solvent Abuse
Case reports suggest an association between maternal solvent abuse and pregnancy loss, intrauterine growth retardation, prematurity, microcephaly, and developmental delay. However, the magnitude of risk remains unknown.
Most infants exposed to drugs in utero will not have physical signs of problems, but there are concerns that fetal drug exposure can lead to behavioral problems later in childhood. To date, no conclusive studies have been able to confirm this link, but it is presumed that at least a portion of these infants are at risk for learning and behavioral problems. Socioeconomic elements that can accompany maternal substance abuse (ie, inadequate parenting skills, poverty, lack of education) may ultimately prove to have as significant an impact on the long-term outcomes for these children as the physiological consequences.
 PATERNAL EXPOSURES
PATERNAL EXPOSURES
Although information is available regarding maternal exposures during pregnancy, limited information exists surrounding outcomes from paternal exposures. There is concern that environmental exposures could affect the egg or sperm cells. However, studies of such mutagenic exposures do not reveal an increased risk of birth defects; damage of the germ cells appears only to affect the fertility of those cells. Semen studies of men exposed to known teratogens did not suggest an increased risk of malformations. In addition, concentration of the agent in semen does not appear to have systemic effects in women and therefore does not affect the pregnancy, except when an infection is transmitted to the mother through the semen.
COUNSELING PATIENTS ABOUT TERATOGENS
 COUNSELING PARENTS
COUNSELING PARENTS
Most pregnancies in the United States are not planned and a majority of women will have been exposed to some type of agent during their pregnancy. When a child is born with an anomaly, families are therefore likely to focus on a putative teratogen as the cause and frequently turn to their physicians for advice and counsel.
Preconceptional Counseling
Drug exposures early in pregnancy may result in a higher potential for teratogenicity because of the critical period of tissue differentiation and organ system development. Many women are not yet aware that they are pregnant for much of this critical period; preconceptional counseling is critical for women during their reproductive years. Because more than 50% of pregnancies in the United States are not planned, this counseling should not be limited to only those anticipating a pregnancy.
Women with chronic medical conditions must be counseled carefully about potential pregnancies. Correction or improvement of the condition before conception can improve maternal health during pregnancy, resulting in a positive fetal outcome. For example, strict periconceptional control of conditions such as diabetes mellitus and phenylketonuria is known to improve pregnancy outcomes. Many women are concerned that their medications pose a risk to the fetus and they stop taking them, which can have an adverse effect on the fetus.
Counseling women regarding preconceptional use of multivitamins containing folic acid, which may reduce the risk of neural tube defects (NTD), is also important. The Centers for Disease Control and Prevention (CDC) recommend that all women of childbearing years take a daily multivitamin with at least 0.4mg (400 mcg) of folic acid. Women who have had a child with an NTD should take 4.0 mg (4000 mcg) of folic acid daily prior to conception and through the first trimester. For more information on folic acid during pregnancy visit the CDC Web site at http://www.cdc.gov/ncbddd/folicacid/.
Counseling Pregnant Patients
The basic approach to counseling a patient about the risk of exposures during pregnancy includes discussion of the 3% background risk of congenital malformations. One of the most important goals of patient counseling is to avoid unnecessarily alarming the patient. The Organization of Teratology Information Specialists (OTIS) provides referrals to local Teratology Information Services, which are comprehensive and multi-disciplinary resources for medical consultation on prenatal exposures. For a referral to a local service, call 1-866-626-OTIS(6847), or visit the OTIS Web site at http://otispregnancy.org/otis_find_a_tis.asp.1-3
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


