KEY POINTS
• Screening for gestational diabetes mellitus (GDM) should be undertaken at 24 to 28 weeks’ gestation. Patients at high risk for GDM (e.g., obesity, prior history of GDM or glucose intolerance, fetal macrosomia, history of polycystic ovarian syndrome, presence of glycosuria, strong family history of type 2 diabetes) should be screened at the first prenatal visit.
• Pregnant women with diabetes may experience periods of hyperglycemia, which can result in fetal hyperglycemia and hyperinsulinemia that is associated with excessive fetal growth and other morbidities.
• Poor glycemic control during early pregnancy (organogenesis) is associated with an increased risk for miscarriage. Congenital malformations occur two to four times more frequently in infants born to women with pregestational diabetes. Cardiac, central nervous system (CNS), and skeletal malformations are most common, but there are no malformations that are pathognomonic for diabetes.
• Preconceptional counseling and medical management should be offered to all patients with pregestational diabetes or glucose intolerance in order to optimize perinatal outcome with pregnancy.
• Women with diabetes complicated by vascular disease (especially nephropathy and retinopathy) are at greatest risk for poor perinatal outcome with an increased risk for preeclampsia, preterm delivery, and fetal growth restriction (FGR).
Background
Diabetes mellitus (DM) is the most common medical complication of pregnancy. It is a metabolic disorder characterized by hyperglycemia resulting from relative deficiency of pancreatic insulin production, limited insulin release in response to a carbohydrate (CHO) challenge, or impaired effect of insulin at the cellular level. Clinically, it manifests as hyperglycemia and increased fat and protein catabolism. This may result in ketosis, which progresses to ketoacidosis. Epidemiologic studies have shown that the prevalence of DM diagnosed among women of childbearing age has increased dramatically in the United States and that a substantial proportion of the population has undiagnosed DM, abnormal fasting glucose levels, or impaired glucose tolerance (1). The cause is multifactorial and includes genetic and environmental contributing factors.
White Classification of DM during Pregnancy
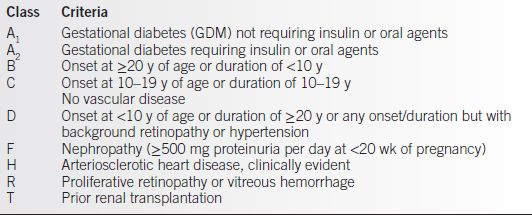
• The White classification was first proposed by Priscilla White, M.D. in 1932. This classification is only used during pregnancy and is based on the duration of diabetes and the secondary vascular and other end-organ complications (2). Although it is somewhat descriptive of risk and health status, it does not differentiate by underlying pathophysiology (Table 17-1).
• The American Diabetes Association Expert Committee categorizes patients by underlying pathogenesis (insulin-deficient type 1, insulin-resistant type 2, and GDM) (3).
• American Diabetes Association Classification (3)
• Type 1 diabetes results from pancreatic β-cell destruction, usually leading to absolute insulin deficiency (previously known as insulin-dependent DM or juvenile-onset DM). These patients are prone to ketosis.
• Type 2 diabetes may be variably expressed as
 Insulin resistance with relative insulin deficiency or
Insulin resistance with relative insulin deficiency or
 An insulin secretory defect with insulin resistance (previously known as non–insulindependent DM or adult-onset DM)
An insulin secretory defect with insulin resistance (previously known as non–insulindependent DM or adult-onset DM)
 Ketosis is unlikely except with severe illness.
Ketosis is unlikely except with severe illness.
 Obesity, family history, and prior history of GDM are common clinical risk factors.
Obesity, family history, and prior history of GDM are common clinical risk factors.
• Other specific types
 Genetic defects of the β-cell (e.g., maturity-onset DM of the young, MODY)
Genetic defects of the β-cell (e.g., maturity-onset DM of the young, MODY)
 Defects in insulin action
Defects in insulin action
 Diseases of the pancreas
Diseases of the pancreas
• Gestational diabetes is any degree of glucose intolerance with onset or first recognition during pregnancy. The diagnosis is established by glucose tolerance testing.
Table 17-1 White Classification for Pregnant Women with DM
Pathophysiology
Normal Maternal Glucose Regulation (2)
• Maternal tendency to fasting hypoglycemia between meals and overnight while fasting because the fetus continues to draw glucose from maternal bloodstream across the placenta. Peak postprandial blood glucose values rarely exceed 120 mg/dL at any time in normal pregnancy.
• Relative insulin sensitivity improves in the first half of pregnancy. This results in a 50% reduction from prepregnancy insulin requirements and results in increased insulin sensitivity.
• Peripheral resistance to maternal insulin increases due to placental hormones and cytokines (e.g., human placental lactogen, progesterone, prolactin, cortisol, and TNF-α), which progressively increase throughout the second and third trimesters. These cause changes in metabolism and blunt the effectiveness of insulin to lower blood glucose levels (4). This explains why gestationally induced DM is usually not clinically apparent until 24 to 30 weeks of gestation.
• Increased pancreatic insulin release (up to 50% greater by the end of pregnancy) results from maternal insulin resistance.
Fetal Metabolism
• The fetus depends on maternal glucose transported across the placenta as its primary energy source.
• Glucose is continuously transported across the placenta from mother to fetus by facilitated diffusion, whereas insulin does not cross the placenta in significant amounts in either direction (2).
• Fetal glucose levels are approximately 10 to 20 mg/dL lower than maternal (4). By 10 to 12 weeks of gestation, the fetal pancreas is able to produce and secrete both insulin and glucagon (5).
• When postprandial maternal glucose levels surge excessively, the consequent fetal hyper-glycemia leads to episodic stimulation of the fetal pancreatic β-cells to produce excess amounts of insulin (fetal hyperinsulinemia).
• Fetal hyperinsulinemia may promote storage of excess nutrients and fetal macrosomia.
• Fetal hyperglycemia and hyperinsulinemia may have detrimental effects on fetal growth and well-being and are probably responsible for most of the complications incurred by infants of diabetic mothers (IDMs).
Maternal Risks
• With current DM management capabilities, avoidance of pregnancy is rarely recommended in women with pregestational diabetes who have achieved good glycemic control. There has been concern that pregnancy may accelerate the progression of diabetic vascular complications. The relative risk of these complications is directly related to the duration and severity of disease as well as to the degree of metabolic control. With aggressive management of DM, this risk, although increased, is acceptable and possibly no greater than that which would occur over the same 9- to 12-month period (2,6–9).
• Pregnancy may be relatively contraindicated in women with
• Severe cardiovascular complications such as symptomatic coronary artery disease (50% maternal mortality rate) or uncontrolled chronic hypertension
• Severe vascular complications from DM, for example, untreated proliferative diabetic retinopathy or significant renal impairment (serum creatinine greater than 3 mg/dL or creatinine clearance less than 50 mL/min)
• Significant autonomic neuropathy, for example, intractable gastropathy
• Diabetic pregnancies are also at increased risk for various maternal obstetric complications such as
• Accelerated chronic hypertension or preeclampsia
• Preterm delivery
• Maternal birth trauma with vaginal delivery or increased risk for cesarean delivery
• Infectious morbidity (genitourinary tract, endometritis, wound infections)
Fetal Risks
• Diabetic embryopathy (spontaneous abortions and birth defects) occurs in the first 7 weeks of gestation.
• Etiology is probably multifactorial (10).
• Hyperglycemia in early pregnancy increases risk for spontaneous abortions (11,12). Women with tight glycemic control and normal glycosylated hemoglobin (HbA1c) values in early pregnancy have a risk for miscarriage equivalent to that of patients without diabetes (2).
• Other potential teratogens synergistic with hyperglycemia include ketones, inhibitors of somatomedin activity, deficiency of myoinositol, accumulation of sorbitol, and reduced levels of arachidonic acid with overproduction of oxygen free radicals that leads to abnormalities in prostaglandin metabolism. This may result in embryopathy by disrupting the vascularization of developing tissues (13).
• Among the general population, the risk in pregnancy of a major birth defect is 1% to 2%. Among women with overt diabetes before conception, the risk of a fetal structural anomaly is increased up to eightfold. Congenital anomalies account for approximately 50% of the perinatal deaths in IDMs, but there is no anomaly that is pathognomonic for fetal exposure to maternal diabetes (14).
 Two-thirds of anomalies involve cardiovascular (8.5 per 100 live births) or CNS (5.3 per 100 live births).
Two-thirds of anomalies involve cardiovascular (8.5 per 100 live births) or CNS (5.3 per 100 live births).
 Anencephaly and spina bifida occur 13 to 20 times more frequently among IDMs compared to infants born to pregnancies not complicated by diabetes.
Anencephaly and spina bifida occur 13 to 20 times more frequently among IDMs compared to infants born to pregnancies not complicated by diabetes.
 Genitourinary, gastrointestinal, and skeletal defect rates are also increased.
Genitourinary, gastrointestinal, and skeletal defect rates are also increased.
 Small left colon syndrome (transient inability to pass meconium, which resolves spontaneously) is unique to IDMs.
Small left colon syndrome (transient inability to pass meconium, which resolves spontaneously) is unique to IDMs.
 The majority of cases of caudal regression syndrome (sacral agenesis) occur in IDMs. This syndrome consists of a spectrum of structural defects of the caudal region including incomplete development of the sacrum and to a lesser degree, the lumbar vertebrae (2).
The majority of cases of caudal regression syndrome (sacral agenesis) occur in IDMs. This syndrome consists of a spectrum of structural defects of the caudal region including incomplete development of the sacrum and to a lesser degree, the lumbar vertebrae (2).
• Diabetic fetopathy (predominantly growth and metabolic abnormalities) occurs in the second and third trimesters.
• Fetal growth abnormalities:
 Fetal macrosomia (fetal obesity, large for gestational age) is increased threefold in IDMs compared to controls. The fetal macrosomia occurring with DM is believed to be secondary to hyperglycemia (which results in fetal hyperinsulinemia) and various growth factors. Increased subcutaneous fat deposition and organomegaly (liver and spleen primarily) occur, whereas the head and brain remain normal in size. The disproportion in size of the fetal head and the body can result in a traumatic vaginal delivery complicated by shoulder dystocia and attendant risks (e.g., brachial plexus injury, clavicle/humerus fractures). Approximately 70% of fetal growth occurs in the third trimester. These complications may be associated with significant neonatal morbidity.
Fetal macrosomia (fetal obesity, large for gestational age) is increased threefold in IDMs compared to controls. The fetal macrosomia occurring with DM is believed to be secondary to hyperglycemia (which results in fetal hyperinsulinemia) and various growth factors. Increased subcutaneous fat deposition and organomegaly (liver and spleen primarily) occur, whereas the head and brain remain normal in size. The disproportion in size of the fetal head and the body can result in a traumatic vaginal delivery complicated by shoulder dystocia and attendant risks (e.g., brachial plexus injury, clavicle/humerus fractures). Approximately 70% of fetal growth occurs in the third trimester. These complications may be associated with significant neonatal morbidity.
 Fetal growth restriction (FGR) and small for gestational age (SGA) may occur especially in women with underlying vasculopathy (retinal, renal, or hypertension) and may result from compromised uteroplacental blood flow. Concurrent hypertension and fetal structural anomalies may contribute to FGR/SGA.
Fetal growth restriction (FGR) and small for gestational age (SGA) may occur especially in women with underlying vasculopathy (retinal, renal, or hypertension) and may result from compromised uteroplacental blood flow. Concurrent hypertension and fetal structural anomalies may contribute to FGR/SGA.
• Polyhydramnios occurs presumably as a result of fetal osmotic diuresis associated with hyperglycemia.
• Intrauterine death risk may be related to fetal hypoxia, acidosis, or fetal cardiac arrhythmia particularly in the setting of diabetic ketoacidosis (DKA).
• Neonatal metabolic abnormalities are more likely with poor glycemic control (15).
 Neonatal hypoglycemia can lead to neonatal seizures, coma, and brain damage if unrecognized.
Neonatal hypoglycemia can lead to neonatal seizures, coma, and brain damage if unrecognized.
 Polycythemia is due to increased erythropoietin production and red blood cell hyperplasia. Polycythemia can lead to poor circulation and postnatal hyperbilirubinemia.
Polycythemia is due to increased erythropoietin production and red blood cell hyperplasia. Polycythemia can lead to poor circulation and postnatal hyperbilirubinemia.
 Hypocalcemia is secondary to a functional hypoparathyroidism with unclear etiology.
Hypocalcemia is secondary to a functional hypoparathyroidism with unclear etiology.
• Hypertrophic and congestive cardiomyopathies may also occur and are thought to be secondary to fetal hyperinsulinemia. Episodic fetal hypoxia stimulated by hyperglycemia may lead to an outpouring of adrenal catecholamines and may result in fetal hypertension, cardiac remodeling, and hypertrophy (2).
• Respiratory distress syndrome or delayed fetal pulmonary maturity may also occur in pregnancies with poor glycemic control.
• Childhood obesity and glucose intolerance may occur later in life (16,17).
EVALUATION
Pregestational Diabetes and GDM Diagnosed Prior to 20 Weeks
Maternal Evaluation
• The history and physical should be comprehensive.
• Refer to an ophthalmologist for retinal assessment if this has not been completed in the past 6 to 12 months.
• Initial laboratory tests/studies should include
• Routine prenatal blood tests.
• Complete metabolic panel (CMP) with baseline preeclampsia labs including liver function studies (some pregestational patients may have underlying fatty liver changes), BUN, creatinine, and uric acid.
• Thyroid function tests (thyroid-stimulating hormone [TSH], free T4, and free T3 if indicated) are indicated as up to 20% of patients with pregestational diabetes may have thyroid dysfunction. More recent data suggest that trimester-specific TSH cutoffs should be used to identify thyroid dysfunction during pregnancy (TSH >2.5 mIU/L in the first trimester, TSH >3.0 mIU/L in the second and third trimesters).
• Glycosylated hemoglobin (HbA1c).
• Urine tests: urinalysis and culture, 24-hour urine collection for total protein and creatinine clearance. A microalbumin dip <30 mg or 24-hour total protein less than 300 mg and/or creatinine clearance greater than 60 mL/min are normal.
• EKG for women with pregestational DM >10 years’ duration, >35 years of age, or for any patient with history or clinical symptoms of cardiovascular disease (including hypertension).
• Subsequent laboratory tests to consider include
• Prenatal genetic screening options as otherwise indicated. This includes first-trimester early aneuploidy screening (11 to 14 weeks), Tetra marker analyte screening (15 to 20 weeks), and maternal blood–free fetal DNA screening. Maternal serum α-fetoprotein (MSAFP), unconjugated estriol (uE3), and inhibin A that are components of some second-trimester Down syndrome screening tests are significantly reduced in women with diabetes such that the MoM values must be adjusted. Maternal DM does not increase the risk for fetal aneuploidy.
• Serial HbA1c assessments every 4 to 8 weeks.
• Repeat 24-hour urine collection in the second and third trimester if abnormal on initial evaluation (e.g., if the 24-hour total protein is greater than 300 mg or creatinine clearance is less than 50 mL/min) or if increased proteinuria is noted on urine dipstick evaluations.
• Common maternal complications to monitor on follow-up visits include
• Hypertension (greater than 140/90 mm Hg)
 Chronic if identified in the first 20 weeks’ gestation
Chronic if identified in the first 20 weeks’ gestation
 Possibly preeclampsia if identified in the latter part of pregnancy
Possibly preeclampsia if identified in the latter part of pregnancy
• Discovery of nephropathy, retinopathy, gastropathy, or neuropathy
• Preterm labor +/– polyhydramnios
• Increased risk for hypoglycemia and DKA
Fetal Evaluation
• Growth and Development:
• Dating ultrasound to confirm gestational age as soon as possible.
• Ultrasound at 11 to 14 weeks’ gestation for nuchal translucency (if indicated).
• Ultrasound at 18 to 20 weeks’ gestation to evaluate fetal morphology.
• Fetal echocardiogram at 22 to 23 weeks’ gestation to exclude congenital heart disease.
• Ultrasound at 28 to 32 weeks’ gestation to assess fetal growth (macrosomia or FGR) and to assess amniotic fluid volume (polyhydramnios or oligohydramnios).
• Ultrasound prior to delivery (38 weeks’ gestation) for estimated fetal weight (EFW) may be helpful in delivery planning.
• Fetal Well-Being:
• Antenatal fetal testing is recommended as surveillance for potential uteroplacental insufficiency and fetal compromise in diabetic pregnancies requiring insulin or oral agent therapy for glycemic control.
• Fetal kick counts starting at 26 to 28 weeks’ gestation.
• Fetal testing options are contraction stress test (CST), nonstress test (NST), biophysical score (BPS), and modified biophysical profile (MBPP = BPS + amniotic fluid volume).
• Testing should start no later than 32 to 34 weeks’ gestation and should continue until delivery.
 Fetal testing should be individualized. Patients with vasculopathy (e.g., class F, R, H, T) or who develop complications during pregnancy (e.g., hypertension, very poor glycemic control, or FGR) may benefit from fetal testing earlier in pregnancy (26 to 32 weeks).
Fetal testing should be individualized. Patients with vasculopathy (e.g., class F, R, H, T) or who develop complications during pregnancy (e.g., hypertension, very poor glycemic control, or FGR) may benefit from fetal testing earlier in pregnancy (26 to 32 weeks).
• The following testing schemes are acceptable:
 CST alternating with MBPP every 3 to 4 days (i.e., on a Monday/Thursday or Tuesday/Friday schedule) has been shown to be an effective method of fetal surveillance for diabetic pregnancies specifically. When CST is contraindicated, is not readily available, or yields an equivocal result, then BPS is an appropriate backup test.
CST alternating with MBPP every 3 to 4 days (i.e., on a Monday/Thursday or Tuesday/Friday schedule) has been shown to be an effective method of fetal surveillance for diabetic pregnancies specifically. When CST is contraindicated, is not readily available, or yields an equivocal result, then BPS is an appropriate backup test.
 CST and BPS alternating every 3 to 4 days.
CST and BPS alternating every 3 to 4 days.
 NST and BPS alternating every 3 to 4 days.
NST and BPS alternating every 3 to 4 days.
 MBPP alone every 3 to 4 days.
MBPP alone every 3 to 4 days.
 Note that the testing schemes of alternating NST/BPS or MBPP alone are most commonly used for class A1, A2, B, and C DM (e.g., diabetes without vascular complications).
Note that the testing schemes of alternating NST/BPS or MBPP alone are most commonly used for class A1, A2, B, and C DM (e.g., diabetes without vascular complications).
Pregestational Diabetes
Maternal Evaluation
• A comprehensive history and physical should be performed.
• Initial laboratory tests should include
• Routine prenatal blood tests.
• HbA1c should be considered if there is any question that DM may be long-standing and undiagnosed.
• Twenty-four-hour urine collection for total protein and creatinine clearance.
• Thyroid function tests (TSH and free T4, plus free T3 if indicated).
 Baseline preeclampsia labs if patient has concurrent chronic hypertension.
Baseline preeclampsia labs if patient has concurrent chronic hypertension.
• Subsequent laboratory tests to consider:
• Serial HbA1c assessments every 4 to 8 weeks can be helpful if compliance with dietary recommendations and self-monitoring of blood glucose (SMBG) is questionable.
• CMP including liver function studies. This is of particular importance when using oral agent therapy in order to rule out underlying liver abnormalities.
• Serial prenatal assessments for
• Preeclampsia
• Preterm labor
• Fetal growth disorders
• Polyhydramnios
Fetal Evaluation
• Growth and Development:
• Ultrasound at 28 to 32 weeks to assess fetal growth (LGA/macrosomia or SGA/FGR) and to assess amniotic fluid volume (polyhydramnios and oligohydramnios).
• Ultrasound prior to the onset of labor for EFW may be helpful in delivery planning. However, EFW values have a variance of ±15% generally and potentially even greater for a macrosomic fetus.
• Antenatal fetal surveillance (using a similar testing scheme as outlined above):
• GDM A1 not requiring insulin or oral agents does not require testing prior to 40 weeks’ gestation unless hypertension, polyhydramnios, or fetal macrosomia is present.
• GDM A2 requiring insulin or oral agents requires testing starting at 32 to 34 weeks’ gestation.
• Fetal kick counts should be encouraged starting at 26 to 28 weeks’ gestation.
DIAGNOSIS
Overt Diabetes
• It is not unusual for undetected, pregestational diabetes to be diagnosed during pregnancy. Any woman with suspected DM when prenatal care begins should be tested immediately to establish the diagnosis. Glycosuria is common but not diagnostic nor necessary for the diagnosis.
• The IADPSG (International Association of Diabetes in Pregnancy Study Group) Guidelines states that any one of the following criteria establishes the diagnosis of pregestational DM during the first trimester (Table 17-2):
• An early HbA1c of 5.7% to 6.5% or an FPG >92 mg/dL and <126 mg/dL is highly suspicious for “prediabetes” and should, preferably, have confirmation by two-step screening or managed as GDM.
• GDM diagnosed at less than 20 weeks’ gestation should be managed as if there was known pregestational DM.
• The American College of Obstetricians and Gynecologists (ACOG) and the National Institutes of Health (NIH) Consensus Panel currently recommend the use of two-step screening and diagnostic criteria. Neither organization endorses the use of the one-step screening and diagnostic criteria recommended by the American Diabetes Association (ADA) and IADPSG.
Table 17-2 Diagnosing Preexisting Diabetes during Pregnancy

Adapted from The International Association of Diabetes and Pregnancy Study Group (18).
Gestational Diabetes Mellitus (GDM)
• GDM, defined as “carbohydrate intolerance of varying degree of severity with onset or first recognition during pregnancy” (19), affects 1% to 10% of all pregnancies and accounts for about 90% of all patients with DM during pregnancy. Specific ethnic groups (Hispanic, African American, Native American, South or East Asian, or Pacific Island ancestry) are at significantly greater risk for GDM. The prevalence of GDM is increasing and varies in direct proportion to the prevalence of obesity and type 2 DM in any given population (19). The definition of GDM applies regardless of whether or not insulin is used for treatment. Importantly, the definition does not exclude the possibility that unrecognized glucose intolerance may have antedated pregnancy.
Screening and Diagnosis for GDM
• Screening for GDM is based on either universal or selective criteria.
• The cost-effectiveness of universal screening of all pregnant women for GDM has been widely debated.
• Selective screening based on historical risk factors alone may identify only 50% of all women with GDM depending on the prevalence of disease in the screened population.
• Selective screening for GDM is accomplished by screening only pregnant women at higher risk for GDM by the following characteristics (19):
• Age greater than 25 years
• Member of a racial or ethnic group with high prevalence of GDM
• Body mass index greater than 25 kg/m2
• History of abnormal glucose tolerance
• Previous history of adverse pregnancy outcomes usually associated with GDM • Known diabetes in first-degree relatives
• Two different protocols are presently advocated for the screening and diagnosis of GDM:
• The one-step diagnostic protocol has been proposed by the World Health Organization (WHO) and IADSPG (18).
 A 2-hour 75-g oral glucose tolerance test (OGTT) is performed with measurement of plasma glucose values at 1- and 2-hour postglucose challenge (unless FBG exceeds 92 mg/dL since this is diagnostic of GDM).
A 2-hour 75-g oral glucose tolerance test (OGTT) is performed with measurement of plasma glucose values at 1- and 2-hour postglucose challenge (unless FBG exceeds 92 mg/dL since this is diagnostic of GDM).
 This protocol has the advantage of a single set of criteria for screening and diagnosis. However, the cost-effectiveness of this protocol has not been confirmed.
This protocol has the advantage of a single set of criteria for screening and diagnosis. However, the cost-effectiveness of this protocol has not been confirmed.
 The patient should perform the test after fasting overnight for at least 8 hours. The current guidelines do not have any requirement for specific dietary preparation prior to the 2-hour 75-g OGTT.
The patient should perform the test after fasting overnight for at least 8 hours. The current guidelines do not have any requirement for specific dietary preparation prior to the 2-hour 75-g OGTT.
 The test is considered abnormal and diagnostic for GDM if any single serum glucose value meets or exceeds the following cutoffs (NIH 20, ADA 21):
The test is considered abnormal and diagnostic for GDM if any single serum glucose value meets or exceeds the following cutoffs (NIH 20, ADA 21):
Fasting plasma glucose (FPG) ≥92 mg/dL (5.1 mmol/L)
One-hour postchallenge ≥180 mg/dL (10.0 mmol/L)
Two-hour postchallenge ≥153 mg/dL (8.5 mmol/L)
 The cutoff values are based on the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study identifying a 1.75-fold increase for selected adverse neonatal outcomes (22). The HAPO study found associations between maternal hyperglycemia and increasing rates of LGA neonates, fetal hyperinsulinemia, neonatal hypoglycemia, and cesarean delivery. Single-step screening using the above cutoff values proposed by the IADPSG may identify a group of patients with “milder” glucose intolerance than those identified with the two-step protocol. It is important to note that the NIH Consensus Panel (2013) determined that there is insufficient evidence-based research to support using this protocol for routine screening at this time (20).
The cutoff values are based on the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study identifying a 1.75-fold increase for selected adverse neonatal outcomes (22). The HAPO study found associations between maternal hyperglycemia and increasing rates of LGA neonates, fetal hyperinsulinemia, neonatal hypoglycemia, and cesarean delivery. Single-step screening using the above cutoff values proposed by the IADPSG may identify a group of patients with “milder” glucose intolerance than those identified with the two-step protocol. It is important to note that the NIH Consensus Panel (2013) determined that there is insufficient evidence-based research to support using this protocol for routine screening at this time (20).
• Two-step diagnosis protocol is commonly used in North America and is endorsed by the American College of Obstetricians and Gynecologists (19) and the NIH Development Conference Statement (20). Note that the two-step approach was not developed to diagnose diabetes in pregnancy per se, but rather, to identify women “at risk” for developing diabetes later in life.
 An initial 1-hour 50-g OGTT is performed.
An initial 1-hour 50-g OGTT is performed.
 No specific dietary preparation or fasting is needed, but it is advisable to instruct the patient to eat at least 3 hours prior to test administration to avoid false-positive results.
No specific dietary preparation or fasting is needed, but it is advisable to instruct the patient to eat at least 3 hours prior to test administration to avoid false-positive results.
 A universally agreed upon cutoff value for the 1-hour 50-g OGTT has NOT been established. Values of 135 and 140 mg/dL are most commonly used:
A universally agreed upon cutoff value for the 1-hour 50-g OGTT has NOT been established. Values of 135 and 140 mg/dL are most commonly used:
– A cutoff of 140 mg/dL has an 80% sensitivity, with 10% to 15% of patients requiring a 3-hour 100-g OGTT.
– A cutoff of 135 mg/dL has greater than 90% sensitivity, with 20% of patients requiring a 3-hour 100-g OGTT (2).
 A 3-hour 100-g OGTT is recommended when the 1-hour 50-g OGTT is abnormal. In preparation for this test, the patient should be instructed to eat more than 150 g of CHO per day for 3 days and to fast for at least 8 hours prior to the test. The test is diagnostic for GDM if any two or more plasma glucose values meet or exceed the following thresholds (19,23):
A 3-hour 100-g OGTT is recommended when the 1-hour 50-g OGTT is abnormal. In preparation for this test, the patient should be instructed to eat more than 150 g of CHO per day for 3 days and to fast for at least 8 hours prior to the test. The test is diagnostic for GDM if any two or more plasma glucose values meet or exceed the following thresholds (19,23):
Fasting plasma glucose ≥95 mg/dL (5.3 mmol/L)
One-hour postchallenge ≥180 mg/dL (10.0 mmol/L)
Two-hour postchallenge ≥155 mg/dL (8.6 mmol/L)
Three-hour postchallenge ≥140 mg/dL (7.8 mmol/L)
 If the 1-hour 50-g OGTT is ≥190 mg/dL, there is limited value to proceeding with the 3-hour 100-g OGTT because more than 95% will be abnormal and a presumptive diagnosis of GDM should be made. Further testing is not required except to exclude a significantly elevated fasting glucose and possible immediate need for insulin therapy. The degree of abnormality on the initial 1-hour 50-g OGTT correlates directly with the risk of having an abnormal 3-hour 100-g OGTT (22).
If the 1-hour 50-g OGTT is ≥190 mg/dL, there is limited value to proceeding with the 3-hour 100-g OGTT because more than 95% will be abnormal and a presumptive diagnosis of GDM should be made. Further testing is not required except to exclude a significantly elevated fasting glucose and possible immediate need for insulin therapy. The degree of abnormality on the initial 1-hour 50-g OGTT correlates directly with the risk of having an abnormal 3-hour 100-g OGTT (22).
 For patients who cannot tolerate the OGTT, an alternative glucose load may be substituted (e.g., 18 Brach’s jelly beans, 400 kcal mixed-meal tolerance test, or Polycose meal tolerance test). Patients with a history of gastric bypass surgery (Roux-en-Y) are not always candidates for testing by OGTT as ingesting glucose may result in dumping syndrome. Alternatively, these patients should be evaluated by several days of glycemic profiling (pre- and postprandial glucose values) by SMBG values. GDM will be diagnosed by persistent hyperglycemia (greater than 30% or more if glucose values exceed target ranges).
For patients who cannot tolerate the OGTT, an alternative glucose load may be substituted (e.g., 18 Brach’s jelly beans, 400 kcal mixed-meal tolerance test, or Polycose meal tolerance test). Patients with a history of gastric bypass surgery (Roux-en-Y) are not always candidates for testing by OGTT as ingesting glucose may result in dumping syndrome. Alternatively, these patients should be evaluated by several days of glycemic profiling (pre- and postprandial glucose values) by SMBG values. GDM will be diagnosed by persistent hyperglycemia (greater than 30% or more if glucose values exceed target ranges).
 Plasma glucose values should be obtained by venipuncture. The glucose meters that are currently available are quite accurate, but the validity of using capillary glucose values for screening and diagnosis has not been confirmed.
Plasma glucose values should be obtained by venipuncture. The glucose meters that are currently available are quite accurate, but the validity of using capillary glucose values for screening and diagnosis has not been confirmed.
 A patient should be classified as having GDM A1 if euglycemia is achieved with nutrition therapy alone, as indicated by greater than 70% to 80% of SMBG values being within normal range. Requirement for additional therapy with insulin or oral agents is indicative of GDM A2.
A patient should be classified as having GDM A1 if euglycemia is achieved with nutrition therapy alone, as indicated by greater than 70% to 80% of SMBG values being within normal range. Requirement for additional therapy with insulin or oral agents is indicative of GDM A2.
TREATMENT
Antepartum Management
Patient Counseling and Education
• Patients with DM, classes B to T, should be counseled regarding maternal–fetal risks. Referral to genetics for counseling should be considered for a significantly elevated firsttrimester glycosylated hemoglobin (HbA1c ≥ 10%), exposure to potential teratogens (e.g., oral hypoglycemics, angiotensin-converting enzyme [ACE] inhibitors, angiotensin receptive blockers [ARBS], and cholesterol-lowering statins), or other usual obstetrical and medical indications.
• Team management goals should be reviewed:
• Identification and roles of team members
• Time commitments regarding appointments
• Maternal–fetal testing required
• Self-monitoring of blood glucose (SMBG)
• Medication management
• Nutrition management
• Physical activity/exercise
• Stress management
• Communication with health care providers (contact person for emergencies)
Medical Nutrition Therapy (MNT)
• Medical nutrition therapy (MNT) is the mainstay of therapy for all patients with DM. Nutrition therapy will successfully optimize glycemic control in approximately 80% to 90% of patients with GDM. Unfortunately, there is limited evidence-based research to endorse one dietary plan versus another in the management of DM during pregnancy, and some have questioned the need for “low-carbohydrate diet” in the treatment of GDM (25). A registered dietitian (RD) should initiate MNT within 1 week of diagnosis and monitor the patient’s progress.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


