Endocrine Disorders and Tumors
Thyroid Gland
Diseases of the thyroid gland are uncommon conditions in children occurring in 37 of 1,000 school-aged children in the USA.1 Most of these lesions are diffuse gland hypertrophy or simple goiters. Thyroiditis is the second most common abnormality, followed by thyroid nodules and functional disorders. The incidence of thyroid cancer is 0.54 per 100,000, though it is more common with multiple endocrine neoplasia (MEN) syndromes and radiation exposure.2 Operative management plays a role in many of these conditions.
Embryology and Physiology
Thyroglobulin is recognized histologically as colloid. Thyroid hormone is synthesized at the interface between the follicular cell and thyroglobulin. The first step in thyroid synthesis is the iodination of tyrosine molecules, which are then coupled, and form the thyroid hormones thyroxine (T4) and triiodothyronine (T3). T4 and T3 are produced at a ratio of ~4 : 1. T3 is more potent than T4 on the target cell. Both T3 and T4 enter the target cell at which point all T4 is converted to T3. T3 then enters the nucleus of the target cell and the T3 molecule interacts with nuclear thyroid receptors. This receptor–T3 complex binds to DNA to regulate genetic transcription.3 Thyroid hormone increases cellular oxygen consumption and basal metabolic rate, stimulates protein synthesis, and influences carbohydrate, lipid, and vitamin metabolism.
Non-neoplastic Thyroid Conditions
Goiter and Thyroiditis
The causes of thyromegaly in one study are listed in Table 76-1.4 Simple adolescent colloid goiters are the most common cause. Physiologically, diffuse thyroid enlargement may be due to a defect in hormone production, related to autoimmune diseases, or a response to an inflammatory condition. Goiters are classified as diffusely enlarged or nodular, and either toxic or euthyroid. Most goiters are euthyroid, and resection is rarely indicated.
TABLE 76-1
Etiology of Thyroid Gland Enlargement (n = 152 Children)
Adapted from Jaksic J, Dumic M, Filipovic B, et al. Thyroid disease in a school population with thyromegaly. Arch Dis Child 1994;70:103–6.
The differential diagnosis for diffuse thyroid enlargement is seen in Box 76-1. Laboratory evaluation should begin with plasma free T4 and TSH levels. With a simple colloid goiter, the patient is euthyroid. Ultrasonography (US) or scintigraphy reveals uniform enlargement, and serum thyroid antibody titers are normal. The etiology of this condition may be an autoimmune process.5 The natural history of colloid goiter is not well known, but one study of adolescents reported that nearly 60% of the glands were normal in size 20 years after diagnosis.1 Exogenous thyroid hormone does not significantly improve resolution of the goiter. In rare cases, resection may be indicated because of size or the suspicion of neoplasia.
Chronic lymphocytic (Hashimoto) thyroiditis is another cause of diffuse thyroid enlargement, occurring most frequently in female adolescents. This condition is part of the spectrum of autoimmune thyroid disorders. It is thought CD4 T-cells are activated against thyroid antigens and recruit cytotoxic CD8 T-cells, which kill thyroid cells, leading to hypothyroidism.6 Children are initially euthyroid and slowly progress to become hypothyroid. However, approximately 10% of children are hyperthyroid, a condition known as ‘hashitoxicosis.’ The thyroid gland is usually pebbly or granular, and may be mildly tender.
Ninety-five per cent of patients with Hashimoto thyroiditis have elevated antithyroid microsomal antibodies or antithyroid peroxidase antibodies. Plasma thyroid hormone levels are normal or low, and TSH levels are elevated in 70% of patients. Thyroid imaging is usually not necessary if clinical and laboratory findings are strongly suggestive of the diagnosis. The radionuclide scan usually shows patchy uptake of the tracer and may mimic the findings in Graves disease or multinodular goiter. The principal ultrasound finding is nonspecific, diffuse thyroid hypoechogenicity. Rarely, autoantibodies cannot be detected and fine needle aspiration is needed to confirm the diagnosis. In as many as one-third of adolescent patients, the thyroiditis resolves spontaneously with the gland becoming normal and the antibodies disappearing. Thus, expectant management should be considered. Exogenous thyroid hormone is administered in the hypothyroid patient. However, in euthyroid children, it is ineffective in reducing the size of the goiter.7
Graves Disease
TSH receptor antibodies are present in more than 95% of patients with active Graves disease, but the inciting event eliciting the antibody response against the TSH receptor is unknown. Infection may produce antibodies that react with the TSH receptor. These antibodies stimulate the thyroid follicles to increase iodide uptake and cyclic adenosine monophosphate production, and induce production and secretion of excess thyroid hormone. Scattered epidemiologic reports of disease clustering supports an infectious etiology for Graves disease.8
Current management includes antithyroid medications, ablation with radioactive 131I, and resection.9 In the USA, most pediatric endocrinologists initiate therapy with methimazole (MTH) or propylthiouracil (PPU) which reduce thyroid hormone production by inhibiting follicle cell organification of iodide and the coupling of iodotyrosines. PPU also inhibits peripheral conversion of T4 to T3, and may be the preferred agent if rapid alleviation of thyrotoxicosis is desired. Both medications possess some immunosuppressive activity as evident by a reduction in antithyroid antibodies. In most cases, MTH is preferred because of its increased potency, longer half-life, and associated improved compliance. The initial adolescent dose is 30 mg once daily, which is reduced if the patient is younger. When the patient becomes euthyroid, as determined by normal T3 and T4 levels, the daily dose of MTH should be reduced to 10 mg. T3 and T4 levels should be monitored. The thyroid gland decreases in size in one-half of patients. Thyroid enlargement with therapy signals either an intensification of the disease or hypothyroidism due to overtreatment.
When treating Graves disease, the goal is to allow natural resolution of the underlying autoimmune process. In general, disease remission rate is approximately 25% after two years of treatment, with a further 25% remission every two years.10 The resolution rate decreases if TSH receptor antibodies persist during and after treatment. The addition of T4 to MTH has had variable results in reducing disease recurrence. However, the use of T4 cannot be recommended in children receiving antithyroid medications.
The thyroid gland must be ablated if resistance or severe reactions to the antithyroid medications develop. Both resection and ablation with radioactive 131I have complications. The advantages of 131I therapy include effectiveness, safety, ease of administration, and relatively low cost.9 Even though the disease recurrence rate is low after 131I treatment, patients risk long-term hypothyroidism. Concerns remain over the possibility of teratogenic or carcinogenic effects of 131I in children and adolescents.11
Either a subtotal or total thyroidectomy is indicated for failure or refusal of medical management, or for airway symptoms (Fig. 76-1). Antithyroid medication should be administered to decrease T3 and T4 levels into the normal range before operation. Alternatively, β-blocking agents, such as propranolol, may be used to ameliorate the adrenergic symptoms of hyperthyroidism. In addition, Lugol’s solution, five to ten drops per day, should be administered for 4 to 7 days before thyroidectomy to reduce the vascularity of the gland.
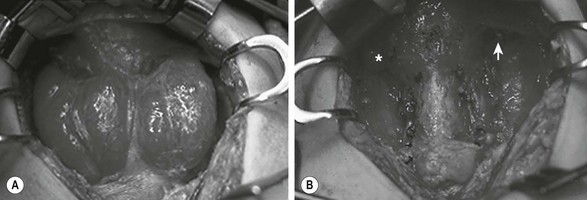
FIGURE 76-1 This teenage girl developed Graves disease and her parents declined radioiodine treatment. (A) The diffusely enlarged, hyperemic thyroid gland is visualized. (B) The thyroid bed is seen after subtotal thyroidectomy. Each upper pole (asterisk, arrow) was left intact.
The incidence of hypothyroidism after subtotal thyroidectomy is variable (10–50%) and the hypothyroidism may be subclinical in up to 45% of children.12 When abnormal TSH levels are considered, the incidence of hyperthyroidism or hypothyroidism is even higher. The rate of recurrent hyperthyroidism is approximately 15%. It is likely the relapse rate increases with time after operation because up to 30% of adult patients exhibit recurrent hyperthyroidism 25 years after their subtotal thyroidectomy.13
Neoplastic Thyroid Conditions
Thyroid Nodules
Thyroid nodules are uncommon in children, but have a 20–25% incidence of malignancy.14,15 Appropriate and prompt evaluation and management are important to decrease disease progression. Various pathology is noted in children who undergo an operation for thyroid nodules (Table 76-2). Other diagnostic possibilities for thyroid nodules include cystic hygroma, thyroglossal duct remnant, and germ cell tumor.
TABLE 76-2
Diagnosis in 251 Pediatric Patients Treated for Thyroid Nodules

Data from Desjardins JG, Khan AH, Montupet P, et al. Management of thyroid nodules in children: A 20-year experience. J Pediatr Surg 1987;22:736–9; Hung W, Anderson KD, Chandra RS, et al. Solitary thyroid nodules in 71 children and adolescents. J Pediatr Surg 1992;27:1407–9; Yip FWK, Reeve TS, Poole AG, et al. Thyroid nodules in childhood and adolescence. Aust N Z J Surg 1994;64:676–8.
As a result of the increased risk of malignancy in younger children, excision of thyroid nodules in prepubescent children is recommended. The natural history of benign lesions in younger children is unknown, and the safety of nonoperative treatment has not been demonstrated. Based on these data, the current recommendation for management of all thyroid nodules in children younger than age 13 is resection. Preoperative ultrasound and thyroid scintigraphy aid in determining the location.14,16
The adolescent spectrum of thyroid disease is similar to that of adults. Thus, fine-needle aspiration is acceptable in evaluating thyroid nodules in this population.17,18 The incidence of malignancy in thyroid nodules in patients age 13 to 18 years is approximately 10%.19 Benign nodules in adolescent patients can be followed with serial examinations and ultrasound. Exogenous thyroid hormone to suppress benign thyroid nodules has not been shown to alter their natural history. Excision should be performed if the nodule is malignant, has indeterminate cytology, increasing size, compressive symptoms, or cosmetic concerns.16 If a cystic thyroid lesion disappears after aspiration, excision can be deferred. If the lesion recurs, it should be removed. Even though cyst fluid can be sent for cytologic analysis, the sensitivity of this test for determining the presence of cancer in children is unknown. Lobectomy can be performed if malignancy is not confirmed preoperatively. If there is a family history, a radiation history, bilateral disease, atypia or suspicious histology, or the lesion is >4 cm, thyroidectomy is recommended.16
Thyroid Carcinoma
Ten per cent of malignant thyroid tumors occur in children, representing about 3% of all pediatric malignancies in the USA. The incidence of childhood thyroid malignancy has decreased in most parts of the world since the mid-1970s due to the reduced use of radiation to treat benign head and neck diseases. However, the incidence may be increasing, perhaps due to improved detection. Overall, mortality remains stable.20
Pediatric thyroid cancer is found more commonly in Caucasians (86%), adolescents (94%), and females (81%). At the time of diagnosis, thyroid cancer is limited to the thyroid gland (42%) or found in the regional lymph nodes (46%). 20 In comparison to adults, children with thyroid carcinoma present with more advanced-stage disease and a higher incidence of lymph node and pulmonary metastases, but have a lower mortality rate.21 Compared to adolescents, prepubertal children have a greater degree of extrathyroid extension, lymph node involvement, and lung metastases at diagnosis.22
Exposure to radiation is a significant risk factor for developing thyroid cancer. A 62-fold increase in thyroid tumors was noted in the Republics of Belarus and Ukraine after the 1986 Chernobyl nuclear power plant catastrophe.23 The tumors were particularly aggressive with increased tumor spread, local invasion, and nodal metastases. The use of radiation for diagnostic purposes has also been linked to increased childhood cancers. It is estimated computed tomography (CT) scans increase risk of malignancy as high as one fatal cancer per 1000 CT scans performed.24,25
Treatment of previous malignancies is another risk factor for thyroid carcinoma. Thyroid cancers constitute about 9% of second malignancies.26 Hodgkin lymphoma is the most common malignancy associated with a subsequent thyroid cancer, followed by non-Hodgkin lymphoma, and leukemia.27 Whereas most thyroid second neoplasms follow previous radiation exposure to the neck, alkylating agents also predispose to thyroid cancer. The mean age at diagnosis of thyroid second neoplasms is 20 years, demonstrating the importance of careful surveillance for second malignancies.
Various molecular biologic events may account for the disparity in behavior of the different histological subtypes of thyroid cancer.28 RAS proto-oncogene mutations are found in about 20% of papillary tumors and 80% of follicular tumors.29 Other studies report RAS is frequently activated in benign follicular adenomas, suggesting this genetic event occurs early in the transformation process.30 An activating mutation of the RET proto-oncogene is found in about 35% of papillary thyroid cancers.31 The RET protein is a receptor tyrosine-kinase molecule, which probably functions within the cell to regulate proliferation or differentiation. This protein may be responsible for the development of medullary thyroid carcinoma (MTC). Specific point mutations are associated with the MEN type 2 (MEN 2A, MEN 2B) syndromes and familial MTC (FMTC). In addition, as many as 40% of patients with sporadic MTCs possess RET mutations.32
Family history is an important factor in pediatric thyroid cancer. Patients with familial nonmedullary thyroid cancer have more aggressive tumors with increased rates of extrathyroid extension, lymph node metastases, and frequently show the phenomenon of ‘anticipation’ (earlier age at disease onset and increased severity in successive generations).33
Thyroid carcinoma usually presents clinically as a thyroid mass, sometimes with enlarged cervical lymph nodes. Regional lymph node metastases are present in three of four children when the disease is first detected (Table 76-3).34,35 Diagnosis of thyroid carcinoma requires appropriate imaging and histological examination. Ultrasound is the diagnostic modality of choice for thyroid lesions with some authors creating Thyroid Imaging Reporting and Data System (TIRADS) to uniformly classify ultrasound characteristics of thyroid nodules.36 In young children, excision is recommended for thyroid nodules after ultrasound evaluation of the thyroid and lymph node basin. In adolescents, ultrasound and ultrasound-guided fine needle aspiration (FNA) is recommended as these patients’ course tends to most resemble adult thyroid disease. US-guided FNA is safe and effective in adolescents when done in experienced centers.16–18,37 As pulmonary metastases are frequent, a preoperative chest radiograph should be obtained in these patients.
TABLE 76-3
Clinical Aspects of Differentiated Thyroid Cancer in Children from Seven Large Pediatric Series133,134
Data from: A, Harness JA, Thompson NW, McLeod MK, et al. Differentiated thyroid carcinoma in children and adolescents. World J Surg 1992;16:547–5542; B, Samuel AM, Sharma SM. Differentiated thyroid carcinomas in children and adolescents. Cancer 1991;67:2186–90; C, Zimmerman D, Hay ID, Gough IR, et al. Papillary thyroid carcinoma in children and adults: Long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery 1988;104:1157–63; D, La Quaglia MP, Corbally MT, Heller G, et al. Recurrence and morbidity in differentiated thyroid carcinoma in children. Surgery 1998;104:1149–56; E, Ceccarelli C, Pacini F, Lippi F, et al. Thyroid cancer in children and adolescents. Surgery 1988;104:1143–8; F, Schlumberger M, De Vathaire F, Travagli JP, et al. Differentiated thyroid carcinoma in childhood: Long-term follow-up in 72 patients. J Clin Endocrinol Metab 1987;65:1088–94; G, Hogan A.R, Zhuge Y, Perez, EA, et al. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res 2009;156:167–72.
Treatment for thyroid cancer in children is total or subtotal thyroidectomy. Thyroid cancer is bilateral in up to two-thirds of cases. Approximately 80% of tumors exhibit multifocality. While lobectomy with isthmus resection may be sufficient for isolated lesions, most pediatric surgeons believe total or near-total thyroidectomy should be performed.38,39 No clinical trial has established whether total thyroidectomy, with lymph node dissection if the regional nodes are involved, is better than subtotal thyroidectomy.40 Radioiodine ablative therapy is more effective after removal of the entire gland because functioning thyroid tissue takes up the radionuclide. Surgeons preferring a lesser resection believe differentiated thyroid carcinoma is an indolent disease, and that survival is not related to the extent of gland removal.40,41
If there is regional nodal metastases, a central or modified radial neck dissection is recommended. Prophylactic central node dissection is recommended for T3 or T4 papillary lesions.16 In patients with locally advanced disease, it is imperative to remove as much of the thyroid gland as possible to allow subsequent radioiodine scanning and treatment if the tumor recurs.
Although complications occur less commonly in recent reports, the historical incidence of recurrent laryngeal nerve injury ranges from 0–24%, whereas permanent hypocalcemia occurs in 6–27% of patients undergoing total thyroidectomy.42,43 During dissection, the laryngeal nerve should be identified and protected. When the tumor invades the laryngeal nerve, the nerve can be safely preserved without compromising survival through adjuvant therapy with 131I irradiation to eradicate residual tumor.44 The most reliable way to preserve parathyroid gland function is to identify and preserve the glands at the time of thyroidectomy (Fig. 76-2). If there is apparent devascularization, then one should autotransplant one or two of the glands into the sternocleidomastoid muscle or into the nondominant forearm.45,46
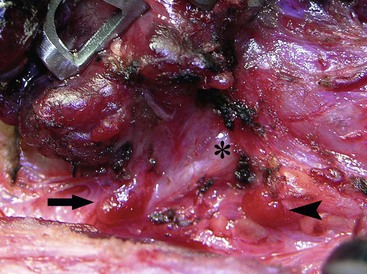
FIGURE 76-2 The relationship of the parathyroid glands to the recurrent laryngeal nerve during prophylactic thyroidectomy for MEN type 2 is seen. The left recurrent laryngeal nerve is marked by an asterisk. The superior parathyroid gland (arrowhead) is typically located posterior to the nerve. The inferior parathyroid gland (arrow) is usually found anterior to the nerve.
The incidence of pulmonary metastases in children at diagnosis is about 6%, but they rarely occur in the absence of significant cervical lymph node metastases.41,47 Pulmonary metastases require treatment with radioiodine. Plain chest films demonstrate the pulmonary disease in only 60% of cases, making scanning with radioiodine necessary. The pulmonary scintiscan can be falsely negative if significant residual thyroid gland remains in the neck.47
In one study, overall survival in non-MTC was 98%.21 A higher rate of recurrence was seen in children who did not receive postoperative 131I than in those who did.21 The time to first recurrence ranged from 8 months to 14.8 years (mean, 5.3 years), thereby emphasizing the importance of long-term follow-up. Prognostic factors associated with recurrence include younger age at diagnosis, histology, capsular or soft tissue invasion, positive margins, and tumor location at diagnosis (thyroid, lymph nodes, lung).21,42 A whole-body 131I scan should be performed approximately six weeks after the initial thyroid resection and followed by therapeutic doses of the radionuclide administered as necessary to ablate residual tissue and treat metastatic disease. Radioactive ablation (RAI) has been shown to decrease the risk of local recurrence, increase the sensitivity of subsequent diagnostic whole-body scans, and improve the utility of serum thyroglobulin as a marker for recurrent or residual disease during long-term follow-up.38 RAI is recommended for all patients with known distant metastases, gross extrathyroidal extension, and primary tumor >4 cm. RAI is recommended for select patients with high risk features such as worrisome histologic subtypes, intrathyroidal vascular invasion, or gross/microscopic multifocal diseases. RAI is not recommended for lesions <1 cm.16 The association of radioactive iodine with second cancers has led to recommendations in the adult literature to avoid radioactive iodine in low risk patients.48
Follow-up for these patients should include physical examination, ultrasound, and thyroglobulin levels. Thyroglobulin can be a useful marker of residual or metastatic thyroid cancer. The plasma level of this protein should be measured yearly, and an elevated value should raise the suspicion of recurrent disease.49 The diagnostic accuracy of this test is decreased in children with residual thyroid tissue or in those who are taking thyroid hormone supplementation. In a long-term study of patients with papillary thyroid cancer, up to 32% of patients had recurrent disease at 40 years follow-up, especially if unilateral lobectomy was performed, emphasizing the need for long-term follow-up.50
MTC accounts for approximately 5% of pediatric thyroid neoplasms. Arising from the parafollicular C cells, MTC may occur either sporadically or in association with MEN 2A, MEN 2B, or the FMTC syndrome. MTC is usually the first tumor to develop in MEN patients and is the most common cause of death in this group. The neoplasm is particularly virulent in patients with MEN 2B and can occur in infancy.51
As with other pediatric thyroid neoplasms, the clinical diagnosis of MTC, not associated with a known familial or MEN syndrome, is usually made only after metastatic spread of the tumor has occurred to the adjacent cervical lymph nodes or to distant sites.52 Resection is the only effective treatment for MTC, underscoring the importance of early diagnosis and therapy before metastases occur.53 For this reason, current management of MTC from MEN 2 and FMTC kindreds relies on the presymptomatic detection of the RET proto-oncogene mutation responsible for the disease. Prophylactic thyroidectomy in young asymptomatic children carrying a mutated allele of the RET proto-oncogene in MEN 2A kindreds has been demonstrated to prevent or cure MTC. In a study of 50 children and teenagers with the RET proto-oncogene mutation who underwent prophylactic thyroidectomy, 66% already had foci of MTC within the thyroid gland.54 However, no child younger than 8 years of age was found to have metastatic disease at surgery. Moreover, when followed for 5 to 8 years, none had evidence of persistent or recurrent disease when screened by physical examination and plasma calcitonin levels obtained after calcium and pentagastrin stimulation. Based on this information, it is recommended that affected children with MEN 2A undergo total thyroidectomy at approximately age 5 years.55–57
Due to the increased virulence of MTC in children with MEN 2B, prophylactic thyroidectomy should be performed at approximately age 1 year. Total thyroidectomy is recommended because of the high incidence of bilateral disease.58 Central lymph node dissection, with removal of lymph nodes medial to the carotid sheaths and between the hyoid bone and the sternum, is likely not necessary except in older children, or when gross lymphadenopathy is discovered at the time of prophylactic thyroidectomy. Early detection by DNA mutation analysis and early operative intervention result in a normal life expectancy.59
Parathyroid Glands
Hyperparathyroidism
The differential diagnosis of hypercalcemia in childhood is shown in Box 76-2. Unlike hypercalcemia in adults, hypercalcemia in children is rarely related to a neoplasm. Rarely, pediatric tumors may secrete a parathyroid-related polypeptide that elevate serum calcium levels. Reported neoplasms producing PTH-like protein include malignant rhabdoid tumor, mesoblastic nephroma, rhabdomyosarcoma, neuroblastoma, and lymphoma. In these patients, the natural PTH level is usually normal or decreased.
Primary Hyperparathyroidism
Primary hyperparathyroidism in childhood typically results from a solitary hyperfunctioning adenoma and more rarely from diffuse hyperplasia of all four glands.60 These lesions are often asymptomatic, but can present with urinary and bone tissue impairment.61 Hyperparathyroidism resulting from hyperplasia in all four glands is a feature of MEN 1. Hyperparathyroidism develops in approximately 30% of MEN 2A patients by their second or third decade of life.62 At the time of prophylactic thyroidectomy for MEN 2, the parathyroid glands can be identified and autotransplanted into the nondominant forearm.45 If hyperparathyroidism develops, a portion of the heterotopic tissue can easily be removed from the forearm.
Primary hyperparathyroidism of infancy is a rare, often fatal, condition usually developing within the first 3 months of life.63,64 Signs include hypotonicity, respiratory distress, failure to thrive, lethargy, and polyuria. The serum PTH level is always elevated, and diffuse parathyroid gland hyperplasia occurs. In about half of the cases, a familial component is found. Early recognition and treatment are essential for normal growth and development.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


