Emergent Endotracheal Intubation
Christopher King
Lara Davidovic Rappaport
Introduction
Few procedures have the same degree of potential impact on the care of a critically ill infant or child as endotracheal intubation. It represents one of the central elements of pediatric resuscitation. Yet the term obviously does not specify a single procedure. It encompasses a variety of techniques that, when successful, lead to the same outcome: insertion of a tube into the trachea for the purpose of delivering positive pressure ventilation to the lungs. By far the most commonly used approach for this procedure is conventional orotracheal intubation via direct laryngoscopy. Mastery of this technique is essential for any practitioner who provides emergency care to children. Blind nasotracheal intubation is a popular choice in the management of adult respiratory emergencies; however, for a variety of reasons discussed later in this chapter, its use is much more restricted among pediatric patients.
The focus of this chapter is emergent intubation, which differs in several respects from elective intubation. The most obvious difference is the importance of time as a constraint during an emergent intubation, because the patient’s condition may be rapidly deteriorating. Furthermore, the patient must always be assumed to have a full stomach, which significantly increases the risk of vomiting and aspiration of gastric contents into the lungs. Depending on the presentation, sedative and paralytic medications may be contraindicated, adding the movements of a struggling child to the list of potential challenges. Finally, compromise due to trauma or an underlying disease process may make the patient more susceptible to adverse physiologic effects of the procedure. These and other factors combine to increase both the complexity and the potential morbidity of emergent endotracheal intubation.
Although it is a skill that requires considerable technical expertise, pediatric intubation is performed by a diverse group of health professionals, including emergency physicians, anesthesiologists, pediatricians, nurse anesthetists, and paramedics. The complexity of this procedure can vary greatly depending on the clinical circumstances. As discussed in Chapter 14, endotracheal intubation can at times prove to be less difficult than properly performed bag-valve-mask (BVM) ventilation. Although the prospect of intubating an apneic child can be a source of anxiety for those who primarily treat adults, certain aspects of pediatric anatomy (e.g., a supple neck, absence of teeth in infants) may make intubating an child less of a challenge. However, all of this is not to say that emergent endotracheal intubation of a pediatric patient can ever be approached casually, because a host of potential hazards must be carefully avoided. This procedure is best performed with an appropriate mixture of confidence and caution to ensure the greatest likelihood for success.
Management of the difficult airway has become an area of intensive study and education and for that reason is the subject of a detailed description in Chapter 17. Nontraditional intubation methods, such as retrograde intubation and tactile intubation, can be effective when used on patients with a known or suspected difficult airway. In addition, improvements in fiberoptic technology have added lighted stylet intubation and flexible fiberoptic intubation to the options available for pediatric patients. Although the majority of endotracheal intubations are managed routinely with orotracheal intubation, proficiency with one or more of the alternative techniques discussed in Chapter 17 can prove invaluable when dealing with the more challenging situations.
Anatomy and Physiology
The most obvious anatomic difference between the airway of an infant or child and that of an adult is size. However, differences also are found in the shape, orientation, and relative positions of structures. These anatomic characteristics change progressively from infancy through adolescence as a result of growth and continuing maturation of the head and neck (Fig. 16.1). Proceeding along the airway from the nose and mouth, several unique features of pediatric anatomy can be identified that have clinical importance in performing endotracheal intubation. Adenoid hypertrophy is common among pediatric patients and may contribute to nasal airway obstruction during manual ventilation. Enlarged adenoids also are more likely to be injured when an endotracheal tube is passed through the nasopharynx, even when gentle pressure is used. Tooth eruption typically begins between 4 and 6 months of age. The emerging teeth of a younger child can potentially be damaged from excessive force applied during direct laryngoscopy. Although infants lack dentition, injury to the alveolar ridge can occur in a similar manner, potentially resulting in abnormalities in subsequent dental development. Primary teeth are shed between 5 and 10 years of age. Accidental dislodgment of one or more primary teeth is not generally harmful to the developing dentition, but an avulsed tooth can be aspirated into the tracheobronchial tree (see “Complications”).
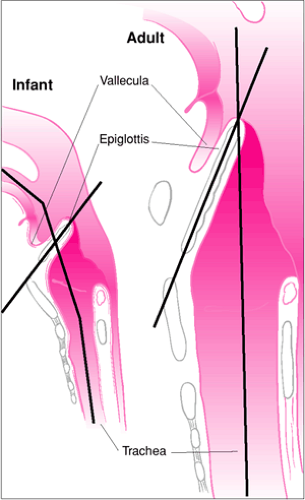
The tongue of a pediatric patient is large in proportion to the rest of the oral cavity (1). With most adults, the mouth will open wide enough to permit the operator to insert a large laryngoscope blade, making retraction of the tongue less difficult. For infants and children, the combination of a small mouth opening and a relatively large tongue frequently requires using a laryngoscope blade that may not easily displace the tongue. Although an often repeated axiom, the pediatric larynx is not anterior but is actually rostral (superior) when compared with an adult’s larynx (2). For example, the larynx of an infant is opposite the C3-C4 interspace, while the larynx of an adult is opposite the C4-C5 interspace (Fig. 16.1). This more superior location of the larynx makes direct visualization of airway landmarks difficult, because the angulation between the base of the tongue and the glottic opening is more acute.
The epiglottis of an adult is broad and has a longitudinal axis that is essentially parallel to that of the trachea. The epiglottis of an infant or young child is relatively narrow, is omega shaped, and has a more acute angle in relation to the axis of the trachea (Fig. 16.2) (3). This angled orientation causes the epiglottis to cover more of the glottic opening and makes retraction with a laryngoscope blade more difficult. Furthermore, the hyoepiglottic ligament, which connects the hyoid bone to the epiglottis, is relatively lax in pediatric patients. In adults, placement of the tip of a curved laryngoscope blade in the vallecula (the space between the base of the tongue and the epiglottis), followed by anterior traction, displaces the hyoid bone anteriorly, which in turn typically retracts the epiglottis because of tension produced in the hyoepiglottic ligament. Laxity in the hyoepiglottic ligament of a child frequently causes this maneuver to be ineffective, requiring direct instrumentation of the epiglottis with the tip of a straight laryngoscope blade to reveal the vocal cords.
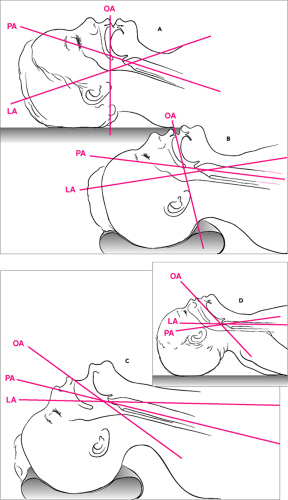
When considering the anatomical structures involved with direct laryngoscopy, it is useful to conceptualize the airway as forming 3 axes—the oral axis, the pharyngeal axis, and the laryngeal (or tracheal) axis (Fig. 16.3). Direct visualization of the anatomic landmarks is greatly facilitated by achieving the most favorable alignment possible of these axes. This is best accomplished by placing the child in the so-called sniffing position, that is, with slight anterior displacement of the neck and extension (rotation) of the head. This is said to be the position normally assumed when a person is “sniffing the air.” With adolescents and older children, achieving the necessary anterior displacement of the neck often requires placing a pad or small towel roll under the head. Because infants and younger children have a relatively large occiput, the neck is already displaced anteriorly when the patient is lying supine, making such additional measures unnecessary. With these younger patients, extension of the head is generally the only maneuver required to achieve the sniffing position. If the occiput is especially pronounced, it may necessary to place a small pad or towel roll under the shoulders to prevent excessive neck flexion.
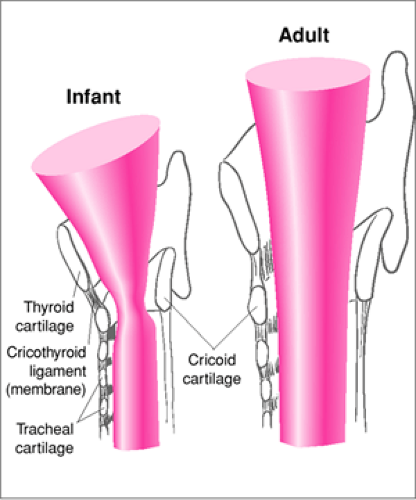
The major cartilaginous skeleton of the larynx is formed by the thyroid cartilage anteriorly, the cricoid cartilage circumferentially, and the arytenoids posteriorly. The overall shape of the airway formed by these cartilages is different in infants than in adults. Because of an underdeveloped cricoid cartilage, the airway of an infant is shaped like a cone, whereas the airway of an adult has a more cylindrical shape (Fig. 16.4). The arytenoids are the cartilaginous attachments of the true vocal cords. During a difficult laryngoscopy, the arytenoids (which appear white compared with the surrounding tissues) may be the only structures of the larynx visualized. In such cases, the endotracheal tube can be guided into the larynx by directing it along the midline anterior to the arytenoid cartilages and thereby through the glottic opening. Additionally, an infant’s vocal cords have a lower attachment anteriorly than posteriorly so that the cords slant “away from” the laryngoscopist, whereas the vocal cords of an adult are essentially perpendicular to the trachea (Fig. 16.2) (3). This angled orientation of the cords increases the likelihood that an endotracheal tube will be held up at the anterior commissure during nasal intubation of an infant or younger child.
The trachea of a newborn is 4 to 5 cm in length and grows to 7 cm by around 18 months of age. The adult trachea is approximately 12 cm long (4). Consequently, movement of the endotracheal tube of only 2 cm within the trachea of an infant may result in endobronchial intubation or tracheal extubation. It is therefore crucial to confirm midtracheal placement of the tube and to properly secure the tube so that minimal movement can occur. The narrowest segment of the larynx in an infant or younger child is at the level of the cricoid cartilage,
whereas the adult extrathoracic airway is narrowest at the vocal cords (5). An endotracheal tube that passes through the glottis of an adult fits loosely in the trachea, because the airway beyond has a larger diameter. However, an endotracheal tube that passes easily through the vocal cords of a young infant may fit tightly in the subglottic region, possibly leading to injury over time. By approximately 10 to 12 years of age, the cricoid and thyroid cartilages have matured sufficiently that both the angulation of the vocal cords and the narrowed subglottic area are no longer present.
whereas the adult extrathoracic airway is narrowest at the vocal cords (5). An endotracheal tube that passes through the glottis of an adult fits loosely in the trachea, because the airway beyond has a larger diameter. However, an endotracheal tube that passes easily through the vocal cords of a young infant may fit tightly in the subglottic region, possibly leading to injury over time. By approximately 10 to 12 years of age, the cricoid and thyroid cartilages have matured sufficiently that both the angulation of the vocal cords and the narrowed subglottic area are no longer present.
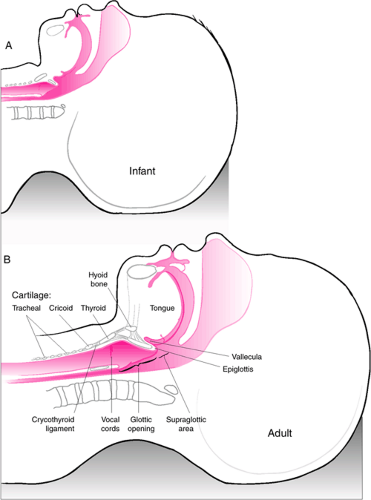 Figure 16.1 Infant versus adult airway anatomy. Important differences regarding endotracheal intubation include the following: (a) the tongue of infant is more rostral and larger relative to the size of the oropharynx, increasing the likelihood of obstruction and making retraction with a laryngoscope blade more difficult; (b) the epiglottis of an infant is more acutely angled over the glottic opening (see also Fig. 16.2); and (c) the larynx of an infant is more rostral (opposite C3-C4) compared with an adult larynx (opposite C4-C5), making the angle of entry into the trachea for tube insertion more acute. |
After birth, the number of alveoli in the lungs increases rapidly. Approximately 20 million alveolar saccules are present at birth (6), increasing to approximately 300 million alveoli by 8 years of age (7). The alveoli grow in both size and number as the child matures, which in turn increases the gas-exchanging surface area of the lung. Therefore, the alveolar surface area of an infant is only one third to one half that of an adult when normalized for body surface. The adult lung also contains small channels that allow ventilation distal to an obstructed bronchus; these pathways are not present in infancy, developing subsequently between the first and second year of life (8). The smaller size of the alveolus and the absence of channels for collateral ventilation combine to increase the risk of developing atelectasis among infants.
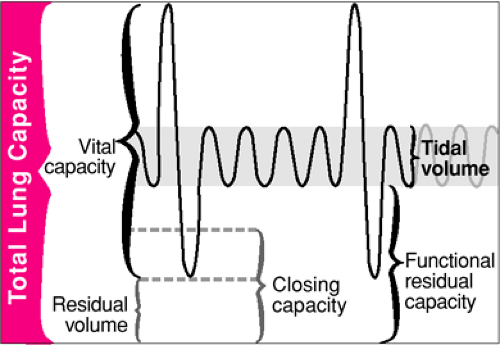
To understand pulmonary ventilation and the pathophysiologic processes that lead to respiratory failure in children, it is useful to review the functional subdivisions of the lungs (Fig. 16.5). The relative size of the lung compartments is approximately constant from infancy through adulthood. The total lung capacity (TLC) is the maximum lung volume that can be achieved during inspiration. The residual volume (RV) is the amount of air remaining in the lung after maximum expiration and equals approximately one fourth of TLC. The functional residual capacity (FRC) is the lung volume at the completion of normal, unforced exhalation. FRC is determined by the balance between the outward stretch of the thorax versus the inward recoil of the lung and normally equals about one half of TLC. As gas is exhaled and lung volumes decrease, the terminal bronchioles are no longer supported by the elastic recoil of the lung and eventually collapse. The lung volume at which this occurs is the closing volume (CV). When CV is reached, alveolar units distal to the collapsed bronchioles do not participate in gas exchange. Lungs of infants and younger children are extremely compliant (i.e., have a low elastic recoil), probably because the elastic fibers are insufficiently developed. This characteristic resembles that of geriatric, emphysematous lungs in which the closing volume is greater than FRC. Consequently, some lung segments will not be ventilated during normal tidal breathing when younger patients lie supine (9,10). This results in an intrapulmonary shunt, which contributes to the rapid desaturation that occurs when ventilation is interrupted.
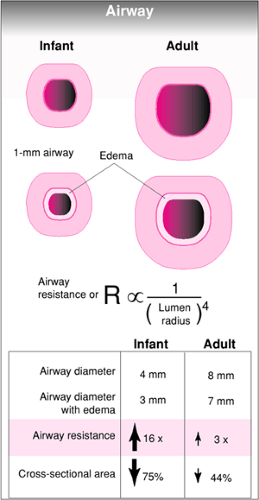
One of the most important physiologic concepts with regard to airway management of a pediatric patient is Poiseuille’s law, which states that the resistance to gas flowing through an airway is inversely proportional to the fourth power of the radius of the airway. In other words, a small decrease in the diameter of an airway results in a large increase in resistance. This can have a profound impact on the work of breathing. Because the airway of a child is smaller to begin with, a decrease in the internal diameter by a given amount (due to edema, obstruction, etc.) results in a greater increase in resistance for pediatric patients than for adults (Fig. 16.6). Furthermore, disease processes that lead to the narrowing of small airways disproportionately increase the work of breathing in infants and children, which accounts for the greater incidence of lower airway obstructive disease seen in this age group.
Because infants and younger children have a highly compliant chest wall and horizontally positioned ribs, thoracic ventilation is relatively inefficient. These patients therefore rely predominately on diaphragmatic breathing (11,12). For this reason, increases in abdominal pressure will significantly compromise ventilation, because movement of the diaphragm is impeded. In addition, infants are predisposed to ventilatory muscle fatigue because they have a lower percentage of slow-twitch, fatigue-resistant muscle fibers in the diaphragm (13,14,15).
Respiratory failure may be defined as the inability of the respiratory system to meet the metabolic demands of the body for the uptake of oxygen and CO2 excretion. Respiratory failure is caused by one of two processes—either failure of the lungs to exchange gas or failure of the respiratory pump to ventilate the lungs. Gas exchange involves conduction of gas through airways, diffusion of gas across the alveoli, and distribution of gas from the pulmonary circulation to the body. Disease processes that affect any of these physiologic mechanisms will impair the gas-exchanging capability of the lungs.
In the majority of patients, the primary disturbance is an alteration in the normal ventilation (V) and perfusion (Q) in the lung, resulting in hypoxemia. The term “respiratory pump” is used when referring to those structures and mechanisms that control ventilation of the lungs (i.e., the nervous system, respiratory musculature, and thoracic cage). In response to changes in pCO2 and pO2, respiratory centers in the brainstem send a signal via the phrenic and intercostal nerves to the respiratory muscles. The resulting contraction of these muscles causes expansion of the thorax and the development of negative pressure within the pleural space. The pressure gradient produced causes gas flow into the lungs. Exhalation occurs passively through elastic recoil of the lungs and chest wall. Failure of gas exchange, through VQ mismatch or intrapulmonary shunting, primarily impairs oxygenation. By contrast, disorders that affect ventilation manifest predominately with hypercarbia as a result of inadequate excretion of CO2. Mild hypoxia also may be present with ventilatory insufficiency, because increased CO2 in the alveolus dilutes available O2.
In the majority of patients, the primary disturbance is an alteration in the normal ventilation (V) and perfusion (Q) in the lung, resulting in hypoxemia. The term “respiratory pump” is used when referring to those structures and mechanisms that control ventilation of the lungs (i.e., the nervous system, respiratory musculature, and thoracic cage). In response to changes in pCO2 and pO2, respiratory centers in the brainstem send a signal via the phrenic and intercostal nerves to the respiratory muscles. The resulting contraction of these muscles causes expansion of the thorax and the development of negative pressure within the pleural space. The pressure gradient produced causes gas flow into the lungs. Exhalation occurs passively through elastic recoil of the lungs and chest wall. Failure of gas exchange, through VQ mismatch or intrapulmonary shunting, primarily impairs oxygenation. By contrast, disorders that affect ventilation manifest predominately with hypercarbia as a result of inadequate excretion of CO2. Mild hypoxia also may be present with ventilatory insufficiency, because increased CO2 in the alveolus dilutes available O2.
Physiologic characteristics of the pediatric cardiovascular system also can have important clinical consequences during endotracheal intubation. For example, infants (and particularly neonates) have an exaggerated response to vagotonic stimuli, due primarily to a relative lack of sympathetic development in the heart (16,17). Consequently, vagal stimulation
can sometimes cause profound bradycardia in these patients (18,19). Furthermore, neonatal lamb studies suggest that immature myocardium has a limited ability to increase stroke volume, and therefore cardiac output primarily depends on heart rate (20,21). The combination of these two factors accounts for the fact that vagal stimuli have a much greater negative impact on cardiac output in younger patients, potentially resulting in significant hypotension. Laryngoscopy can have a wide range of physiologic effects on the cardiovascular system (Table 16.1). Catecholamine release during laryngoscopy can lead to an increase in heart rate and systemic blood pressure,
although this is more common among adults; with pediatric patients, the vagal effects of upper airway instrumentation tend to predominate. Another potential adverse consequence of tracheal intubation relates to the institution of positive pressure ventilation, which may produce hypotension when venous return is diminished as a result of elevated intrathoracic pressure. During spontaneous ventilation, the negative intrathoracic pressure generated favors venous return to the heart. In a hypovolemic patient, a significant fall in cardiac output and blood pressure may occur when spontaneous ventilation is converted to controlled ventilation.
can sometimes cause profound bradycardia in these patients (18,19). Furthermore, neonatal lamb studies suggest that immature myocardium has a limited ability to increase stroke volume, and therefore cardiac output primarily depends on heart rate (20,21). The combination of these two factors accounts for the fact that vagal stimuli have a much greater negative impact on cardiac output in younger patients, potentially resulting in significant hypotension. Laryngoscopy can have a wide range of physiologic effects on the cardiovascular system (Table 16.1). Catecholamine release during laryngoscopy can lead to an increase in heart rate and systemic blood pressure,
although this is more common among adults; with pediatric patients, the vagal effects of upper airway instrumentation tend to predominate. Another potential adverse consequence of tracheal intubation relates to the institution of positive pressure ventilation, which may produce hypotension when venous return is diminished as a result of elevated intrathoracic pressure. During spontaneous ventilation, the negative intrathoracic pressure generated favors venous return to the heart. In a hypovolemic patient, a significant fall in cardiac output and blood pressure may occur when spontaneous ventilation is converted to controlled ventilation.
TABLE 16.1 Physiologic Effects of Laryngoscopy | |
|---|---|
|
Indications
Disease processes and patient presentations that serve as indications for emergent endotracheal intubation are so numerous that it would be beyond the scope of this chapter to discuss all possibilities in detail. Instead, an overview of the most clinically important topics is given. In general, the two main indications for emergent endotracheal intubation are airway obstruction and respiratory failure. There are also specific clinical scenarios that necessitate this procedure. Primary indications for emergent endotracheal intubation of the pediatric patient are listed in Table 16.2.
Airway Obstruction
Partial or complete airway obstruction is most likely to be life-threatening, and therefore to require emergent endotracheal intubation, when the anatomic site of the obstruction is within the trachea superior to the carina. With a more inferior obstruction, only one of the lungs will be involved, and the patient is less likely to have an acutely dangerous respiratory insult, although the process may become more serious with the passage of time. Obstruction that involves the entire trachea is often referred to as “upper airway” obstruction and represents one of the most rapidly evolving potential causes of death in a patient. This is why, as described in Chapter 12, the first step in the “ABCs” of basic life support is to establish a patent airway through proper positioning of the head and neck. When necessary, endotracheal intubation provides a means of securing a “definitive” airway that ensures a reliable conduit for gas exchange.
TABLE 16.2 Indications for Emergent Endotracheal Intubation of Pediatirc Patients | |
|---|---|
|
When the airway is partially obstructed, airflow becomes turbulent, and noisy respirations or stridor can be heard as air passes through the narrowed portion of the airway. Observation of the timing of stridor can aid in diagnosing the location of airway obstruction (22). Inspiratory stridor is common when airway narrowing is located at the supraglottic or glottic airway. Expiratory stridor is more often noted with airway compression below the level of the thoracic inlet. Both inspiratory and expiratory stridor will be heard with fixed lesions, such as subglottic stenosis, that do not change airway diameter with respirations (23). A barking cough is frequently described with subglottic narrowing, as with croup.
The extent of airway obstruction will determine which clinical signs are present. Children with mild obstruction may have stridor but only with increased airflow, as with crying.
Moderate airway obstruction usually causes retractions, tachypnea, tachycardia, restlessness, and confusion. As airway obstruction progresses, small children will manifest paradoxical chest wall movement: with attempted inspiration against the obstructed airway, the diaphragm distends as the abdomen protrudes, and the compliant chest wall retracts. Cyanosis is a late sign of airway obstruction and necessitates emergency intervention. Appropriate treatment for the child with airway obstruction is based on diagnosis, rate of progression of symptoms, and initial response to therapy. Presence of fever suggests an infection. Patients with congenital anatomic abnormalities (e.g., vocal cord paralysis, laryngomalacia, vascular ring, congenital subglottic stenosis, laryngeal web, or laryngeal cyst) will usually manifest stridor at birth.
Moderate airway obstruction usually causes retractions, tachypnea, tachycardia, restlessness, and confusion. As airway obstruction progresses, small children will manifest paradoxical chest wall movement: with attempted inspiration against the obstructed airway, the diaphragm distends as the abdomen protrudes, and the compliant chest wall retracts. Cyanosis is a late sign of airway obstruction and necessitates emergency intervention. Appropriate treatment for the child with airway obstruction is based on diagnosis, rate of progression of symptoms, and initial response to therapy. Presence of fever suggests an infection. Patients with congenital anatomic abnormalities (e.g., vocal cord paralysis, laryngomalacia, vascular ring, congenital subglottic stenosis, laryngeal web, or laryngeal cyst) will usually manifest stridor at birth.
A child with severe airway obstruction rapidly develops pronounced fatigue and becomes unable to continue adequate respiration, so respiratory failure is quickly followed by respiratory arrest. This progression is accelerated by energy-consuming activities such as crying and agitated movements. The operator must therefore make every effort to calm a child who has severe airway obstruction and to avoid actions that increase agitation (forcing an oxygen mask on the child, restraining the child in a supine position on a bed, etc.) However, it should be emphasized that sedatives or narcotics should not be used for this purpose before securing a definitive airway. Using these drugs can lead to suppression of respiratory drive, resulting in hypoventilation or apnea. In fact, obtaining intravenous access may not be appropriate until the patient is moved to a more controlled setting such as the operating room or intensive care unit (ICU). Parents can be enlisted to distract and comfort the patient (3). The child should be maintained in a comfortable position, humidified oxygen delivered via face mask (or simply using “blow-by” tubing if a face mask causes agitation), and protective airway reflexes maintained until the diagnosis is made and any appropriate treatment can be initiated (e.g., nebulized racemic epinephrine).
For any child who presents with airway obstruction and severe respiratory distress, emergency preparations should be made to secure the airway. Since routine direct laryngoscopy may be difficult or impossible because of altered anatomy, assistance should be sought from a qualified anesthesiologist or otolaryngologist when available. The course of action should be carefully planned, including the primary approach (usually conventional orotracheal intubation) and one or more backup approaches (e.g., fiberoptic laryngoscopy or retrograde intubation) (see also Chapter 17). Preparations also should be made for emergency tracheotomy or cricothyroidotomy if personnel capable of performing these procedures are present (see also Chapter 26). A variety of endotracheal tube sizes should be available in case the initially estimated size does not pass through the narrowed airway. Because a surgical procedure may become necessary in this situation, optimal management of the patient would take place in the operating room, assuming that the child’s clinical condition allows sufficient time for transport.
Acquired Airway Lesions
Infection
Acute laryngotracheobronchitis (croup) usually occurs between the ages of 6 months and 3 years. It is characterized by a preceding upper respiratory tract infection; gradual onset of a hoarse, barking cough; stridor; and thin, copious secretions (23). Diagnosis is generally made on clinical grounds but can be confirmed by a funnel-shaped appearance of the glottic and subglottic area on a frontal radiograph of the neck. Inhaled racemic epinephrine usually reverses airway obstruction. Indications for establishment of an artificial airway include cyanosis, fatigue, and frequent need for racemic epinephrine (more often than every 30 minutes). Establishment of the airway is best accomplished in the operating room, where facilities for bronchoscopy and tracheostomy are readily available (23).
Bacterial tracheitis occurs most commonly in infants and toddlers (24). Many patients will initially have croup, followed by the acute onset of stridor, fever, hoarseness, dysphasia, and a brassy cough. The child generally appears quite ill. Suctioning through an artificial airway is frequently necessary because of significant obstruction from the purulent tracheal exudate (25).
Patients with retropharyngeal cellulitis or abscess typically present with one or more of the following: a history of preceding upper respiratory infection or sore throat, dysphasia, drooling, stridor, meningismus, and a toxic appearance. This process usually affects younger children. On a lateral neck radiograph, a widening of the soft tissues between the air column and cervical vertebrae is evident (23), and an air-fluid level associated with an abscess also may be seen. Caution must be exercised with intubation because the normal anatomy of the larynx may be distorted and abscess rupture can occur.
Blunt Trauma
Potential causes of airway obstruction resulting from orofacial trauma include (a) foreign body (e.g., dislodged teeth), (b) laryngeal or tracheal disruption, and (c) external compression of the airway by an enlarging hematoma. Mandibular fractures are commonly associated with trismus, which will impede mouth opening during intubation. Patients may present with hemoptysis, stridor, subcutaneous emphysema, pneumomediastinum, or pneumothorax when an airway injury has occurred (26,27,28,29,30). Evaluation and appropriate management of potential cervical spine injury must always be considered in patients who have significant orofacial trauma.
Burns
Thermal burns in the airway occur from direct flame injury, explosion products, hot gases, and steam. Patients who present with burns of the face or singed facial hair have been exposed to high-temperature gas that should be assumed to have caused a respiratory burn. Thermal injury usually affects the nasopharynx and larynx, but a burn injury below the vocal
cords occurs only infrequently because the temperature of dry gas decreases rapidly in the extrathoracic airway. Patients who have been exposed to high-temperature gases should be observed for evidence of respiratory obstruction or distress (31,32). If progressive respiratory distress develops, intubation is indicated to clear secretions and carbonaceous sputum and to bypass an edematous larynx. It is important to have available smaller-sized endotracheal tubes than the one estimated for age because of the possibility of airway narrowing as a result of laryngeal and tracheal edema.
cords occurs only infrequently because the temperature of dry gas decreases rapidly in the extrathoracic airway. Patients who have been exposed to high-temperature gases should be observed for evidence of respiratory obstruction or distress (31,32). If progressive respiratory distress develops, intubation is indicated to clear secretions and carbonaceous sputum and to bypass an edematous larynx. It is important to have available smaller-sized endotracheal tubes than the one estimated for age because of the possibility of airway narrowing as a result of laryngeal and tracheal edema.
Chemical burns of the airway usually occur in children after ingestion of caustic substances, particularly following emesis and aspiration. The glottis and subglottic regions are most commonly affected, typically exhibiting inflammation, ulceration, and edema. Tracheal intubation is indicated for patients presenting with respiratory distress (23).
Foreign Body Aspiration
Aspiration of a foreign body usually occurs in toddlers from 1 to 3 years of age. Although virtually any small household article may be aspirated (marbles, toys, etc.), the most commonly retrieved object is a peanut. The most likely cause of life-threatening airway obstruction in a child is a piece of hot dog (33,34). If the patient is unable to speak or otherwise phonate, complete tracheal obstruction should be presumed, and emergent laryngoscopy is indicated for foreign body removal and/or possible intubation. A stable patient with partial airway obstruction should not undergo direct laryngoscopy in the emergency department (ED). A foreign body in a mainstem bronchus may produce a ball-valve effect with positive pressure ventilation. An already hyperinflated lung may become further distended, shifting the mediastinum and leading to cardiovascular collapse. Importantly, attempts at endotracheal intubation in a stable child with partial airway obstruction may further advance a foreign body, causing complete obstruction, and the object may not be retrievable. A patient with partial airway obstruction from a foreign body should therefore be given supportive therapy until the object can be removed endoscopically in the operating room. Only when severe dyspnea and cyanosis occur as a result of airway obstruction and respiratory fatigue should immediate laryngoscopy for removal or intubation be attempted in the ED. A complete discussion of the management of airway foreign bodies can be found in Chapter 51.
Respiratory Failure
Respiratory failure is a common indication for endotracheal intubation and mechanical ventilation. As described previously, disease processes that lead to respiratory failure affect either gas exchange in the lungs or the normal function of the respiratory pump (see “Anatomy and Physiology”). Children with respiratory failure resulting from poor gas exchange demonstrate obvious signs of respiratory distress: tachypnea, nasal flaring, intercostal and suprasternal retractions, and use of accessory muscles of respiration. Arterial blood gases or pulse oximetry will reveal hypoxemia well before the onset of hypercarbia. Persistent hypoxemia causes the patient to be tachypneic and agitated. The decision to intubate is based on the presence of physical findings suggesting excessive work of breathing, severe hypoxemia that is unresponsive to supplemental oxygen, and/or an inadequate response of the patient to initial therapeutic interventions. By comparison, patients with respiratory pump failure may not appear greatly distressed. Arterial blood gas analysis will reveal CO2 retention reflecting the degree of ventilatory insufficiency. For these patients, endotracheal intubation should be performed when it is apparent that ventilatory efforts are inadequate or the clinical condition deteriorates.
Impaired Gas Exchange
Obstructive Lung Disease
Bronchiolitis, asthma, and bronchopulmonary dysplasia primarily affect gas flow through the small airways in the lung and are common causes of respiratory failure in children. Bronchopulmonary dysplasia results from unresolved neonatal lung injury, which causes diffuse fibrosis and a decreased number of alveolar units. These patients also have increased small airway resistance, which can often be partially corrected with bronchodilators (35,36). Bronchiolitis is an acute inflammatory disease of the lower respiratory tract that results in obstruction of small airways. Infants who develop bronchiolitis initially manifest symptoms of an upper respiratory tract infection, which may progress to marked respiratory distress characterized by tachypnea, nasal flaring, chest wall retractions, audible wheezing, and irritability. Lungs are hyperinflated, and auscultation reveals wheezing, prolonged expiration, and rales (37). Asthma is a diffuse obstructive pulmonary disease associated with generalized narrowing of the lower airways from mucosal edema, pulmonary secretions, and constriction of bronchial smooth muscle. Patients with status asthmaticus (i.e., asthma that is unresponsive to standard therapy) present with persistent dyspnea, prolonged expiratory wheezing, tachycardia, use of accessory respiratory muscles, and eventually cyanosis. The only absolute indication for emergent intubation of patients with status asthmaticus, bronchiolitis, or bronchopulmonary dysplasia is frank respiratory failure. Aggressive medical management is preferable to endotracheal intubation and mechanical ventilation when feasible, because such patients may be difficult to ventilate and are prone to problems from air trapping (e.g., pneumothorax). However, emergent intubation should be considered whenever the patient has any of the following: (a) a decreased respiratory effort as a result of progressively severe fatigue (38,39), (b) deterioration in mental status (38,40), (c) absence of both breath sounds and wheezing (suggesting minimal gas exchange) (41), (d) cyanosis despite receiving 40% oxygen (41), (e) hypoxemia with a pO2 less than 60 while receiving 6 L/min of O2 (41,42), and (f) hypercapnia with a pCO2
over 65 torr and increasing by more than 5 torr per hour (41). Even with successful intubation, patients may develop reflex bronchospasm, which can lead to worsened hypoxemia and cardiac arrest. The patient’s blood pressure, cardiac rhythm, and oxygen saturation must be carefully monitored during and after this procedure.
over 65 torr and increasing by more than 5 torr per hour (41). Even with successful intubation, patients may develop reflex bronchospasm, which can lead to worsened hypoxemia and cardiac arrest. The patient’s blood pressure, cardiac rhythm, and oxygen saturation must be carefully monitored during and after this procedure.
TABLE 16.3 Conditions Predisposing to a Reduction in Functional Residual Capacity | |
|---|---|
|
Diffuse Pneumonitis
Pneumonitis is one of several conditions that can lead to a reduction in FRC, causing severe impairment of gas exchange, and it is characterized by inflammation of the lung parenchyma (Table 16.3). Infectious pneumonitis may be caused by bacterial, viral, or fungal illness. A chemical pneumonitis may be caused by aspiration, inhalation, or ingestion of toxins (43). These processes result in terminal closure of gas-exchanging units as alveoli collapse (or become fluid filled), producing a large intrapulmonary shunt. Shunting of desaturated blood through the lung causes the patient to manifest significant hypoxemia. Lung compliance is also reduced, leading to an increase in the work of breathing. The decision to perform endotracheal intubation in this situation is based on the response to supplemental oxygen therapy and the degree of respiratory compromise. Patients who are receiving face mask oxygen of 60% or greater but have persistent cyanosis or an oxygen saturation of less than 90% require intubation and positive pressure ventilation. Intubation also should be performed when the patient has an altered mental status or shows signs suggesting excessive work of breathing (e.g., respiratory rate greater than twice normal or accessory respiratory muscle use).
Pulmonary Edema
Pulmonary edema occurs when extravascular fluid accumulates in the lungs. Cardiogenic (hydrostatic) pulmonary edema is caused by impaired left ventricular function as a result of congenital or acquired cardiac disease. Therapy should be directed toward improving cardiac function with pharmacologic or surgical therapies. Noncardiogenic pulmonary edema or respiratory distress syndrome (RDS) occurs following primary lung injury (pneumonia, hydrocarbon aspiration, smoke inhalation, near drowning) or an insult not directly involving the lungs (shock, trauma, sepsis). In this case, pulmonary edema develops from increased permeability of the alveolar-capillary membrane (44). Patients with pulmonary edema exhibit agitation, tachypnea, and hypoxemia from intrapulmonary shunting of venous blood. Hypoxemia may be particularly profound with patients who have noncardiogenic pulmonary edema. The need for endotracheal intubation is based on the patient’s clinical condition and the response to initial therapy for the underlying disease.
Thoracic Trauma
The incidence of acute respiratory failure after chest trauma is approximately 10%, with motor vehicle crashes accounting for the majority of cases (45). Two primary causes of impaired gas exchange in this situation are pulmonary contusion and flail chest. Pulmonary contusion occurs when the lung experiences significant force from blunt trauma to the chest wall. Children are particularly susceptible to this injury because they have increased chest wall compliance and reduced protection from the ribs. Hypoxemia occurs with pulmonary contusion when alveoli collapse and fill with fluid and/or blood. Flail chest, a less common injury among children as compared with adults results from multiple rib fractures and causes a disruption in chest wall integrity. A free-floating portion of the chest wall (the flail segment) moves paradoxically with respiration (i.e., inward with inspiration and outward with expiration). This paradoxical movement leads to atelectasis and VQ mismatching of the underlying lung parenchyma, which often results in significant hypoxemia. Patients with a flail chest frequently have a coexisting pulmonary contusion.
Respiratory Pump Failure
As described previously, the respiratory pump refers to the bellows function of the chest and muscles which moves gas through the conducting airways (see “Anatomy and Physiology”). The brain, spinal cord, peripheral nerves, neuromuscular junction, and muscles make up the five anatomic components necessary for normal function of the respiratory pump. The pump is primarily controlled in the brainstem through the input of chemoreceptors sensitive to PaCO2, PaO2, and pH and through input from the lung regarding airway irritation and stretch applied to the intercostal muscles. Respiratory pump failure may result from muscle weakness or lack of respiratory drive. Endotracheal intubation should be considered whenever a patient manifests the following: (a) frequent episodes of apnea that resolve only with significant stimulation or are associated with hypoxemia and/or bradycardia, (b) a pCO2 increasing at greater than 5 torr per hour, (c) arterial pH less than 7.25, and (d) insufficient strength to generate a cough or gag.
Apnea
Apnea is one of the most common forms of respiratory pump failure among pediatric patients. Risk factors include
prematurity, cardiac or pulmonary disease, respiratory infection, brain injury, gastroesophageal reflux, sepsis, and drug ingestion (Table 16.4). Newborn or premature infants do not display the same increased ventilatory drive from hypoxemia or hypercarbia as adults (46,47,48), and they commonly develop apnea in response to an increased respiratory load (see “Anatomy and Physiology”). Viral infections, particularly infection with a respiratory syncytial virus, can cause central apnea in infants. Infants with cyanotic congenital heart disease have chronic hypoxemia and do not express the normal increased ventilatory drive in response to lower oxygen tension (49). Patients with underlying chronic pulmonary disease (e.g., bronchopulmonary dysplasia) may have chronic CO2 retention and an abnormal sensitivity to increasing CO2 (50). The brain-injured patient most often develops hyperventilation or abnormal respiratory patterns that lower arterial CO2, such as Cheyne-Stokes respiration, although apnea also commonly occurs with injury to the brainstem. Indications for emergent intubation of these patients include (a) impairment or loss of protective airway reflexes, (b) prolonged seizures, and (c) progression of the brain injury to the point of causing abrupt onset of hypoventilation or apnea.
prematurity, cardiac or pulmonary disease, respiratory infection, brain injury, gastroesophageal reflux, sepsis, and drug ingestion (Table 16.4). Newborn or premature infants do not display the same increased ventilatory drive from hypoxemia or hypercarbia as adults (46,47,48), and they commonly develop apnea in response to an increased respiratory load (see “Anatomy and Physiology”). Viral infections, particularly infection with a respiratory syncytial virus, can cause central apnea in infants. Infants with cyanotic congenital heart disease have chronic hypoxemia and do not express the normal increased ventilatory drive in response to lower oxygen tension (49). Patients with underlying chronic pulmonary disease (e.g., bronchopulmonary dysplasia) may have chronic CO2 retention and an abnormal sensitivity to increasing CO2 (50). The brain-injured patient most often develops hyperventilation or abnormal respiratory patterns that lower arterial CO2, such as Cheyne-Stokes respiration, although apnea also commonly occurs with injury to the brainstem. Indications for emergent intubation of these patients include (a) impairment or loss of protective airway reflexes, (b) prolonged seizures, and (c) progression of the brain injury to the point of causing abrupt onset of hypoventilation or apnea.
TABLE 16.4 Differential Diagnosis of Apnea | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Patients with systemic infection have stress-related release of catecholamines, glucagon, and cortisol, which contribute to a hypermetabolic state. The normal response to infection is hyperventilation, although overwhelming sepsis usually leads to respiratory depression (51). When a child with sepsis fails to hyperventilate, incipient respiratory failure should be suspected. Temperature has a direct effect on ventilatory drive. Hyperventilation usually accompanies both heat and cold stress, but deep accidental hypothermia can profoundly depress ventilatory drive. Infants are particularly prone to develop apnea in response to hypothermia. Finally, numerous drugs administered therapeutically or ingested accidentally may suppress normal respiratory efforts. These include opioids and other analgesics, benzodiazepines, and barbiturates. When even small doses of these medications are administered to an acutely ill patient, the effects on respiratory drive may cause hypoventilation or apnea.
Spinal Cord Trauma
Respiratory motor deficits are directly related to the level of spinal injury. High cervical cord injuries (C1-C2), which children are especially prone to sustain, result in apnea and early death without respiratory support. Injury to the middle cervical cord (C3-C5) results in loss of diaphragmatic, intercostal, and abdominal muscle function (52). Accessory muscles of inspiration in the neck and shoulders remain intact, but respiratory insufficiency rapidly develops because these accessory muscles are inadequate to maintain gas exchange. Injury to the spinal cord below the level of C5 may lead to respiratory failure through the development of neurogenic pulmonary edema, but respiratory muscle function is usually adequate to maintain ventilation. Although spinal cord injuries are rare in children, a high index of suspicion should be maintained for all patients with severe head and neck trauma, especially those manifesting coma, flaccidity, hypotension, or hypoventilation.
Neuromuscular Diseases
Many peripheral neuromuscular diseases are complicated by respiratory failure: tetanus, poliomyelitis, Guillain-Barré syndrome, myasthenia gravis, botulism, and myopathies involving the skeletal muscles. Tetanus is now rare because of widespread immunization. Cases of neonatal tetanus, which usually result from contamination and infection of the umbilicus, are still seen occasionally. Laryngospasm and/or spasm of the
respiratory muscles may lead to inadequate ventilation (53,54). Poliomyelitis is caused by an acute viral infection of the CNS, which, in severe cases, results in muscle paralysis and associated respiratory failure. In the United States, sporadic cases are seen among immunocompromised patients exposed to live attenuated virus used for active immunization (55). Guillain-Barré syndrome is an acute inflammatory peripheral neuropathy of unknown etiology that affects both adults and children. Patients typically develop progressive muscle weakness, which is most severe in the lower extremities. Approximately 20% of children with Guillain-Barré syndrome ultimately develop respiratory failure (56). Myasthenia gravis results from the production of antibodies to the acetylcholine receptor, leading to dysfunction of signal transmission at the neuromuscular junction. The disease takes several forms in the pediatric population, each with a unique pathogenesis and clinical picture. Weakness is apparent soon after birth with both the congenital and the neonatal forms of myasthenia and develops later in childhood with juvenile myasthenia (57). Botulism is an acute paralytic disorder caused by ingestion of neurotoxin released by Clostridium botulinum. The toxin is found in food that is contaminated with the organism and processed under anaerobic conditions (58). Patients who ingest the toxin manifest generalized muscle weakness within 36 hours. Infant botulism is a form of the disease unique to children under 9 months of age. The organism is ingested in vivo and colonizes the gastrointestinal tract of the infant, leading to a slow release of toxin. These patients most commonly present between 2 and 4 months of age and manifest poor feeding, constipation, lethargy, and generalized hypotonia. In severe cases, these infants may require emergent intubation and mechanical ventilation (59,60,61).
respiratory muscles may lead to inadequate ventilation (53,54). Poliomyelitis is caused by an acute viral infection of the CNS, which, in severe cases, results in muscle paralysis and associated respiratory failure. In the United States, sporadic cases are seen among immunocompromised patients exposed to live attenuated virus used for active immunization (55). Guillain-Barré syndrome is an acute inflammatory peripheral neuropathy of unknown etiology that affects both adults and children. Patients typically develop progressive muscle weakness, which is most severe in the lower extremities. Approximately 20% of children with Guillain-Barré syndrome ultimately develop respiratory failure (56). Myasthenia gravis results from the production of antibodies to the acetylcholine receptor, leading to dysfunction of signal transmission at the neuromuscular junction. The disease takes several forms in the pediatric population, each with a unique pathogenesis and clinical picture. Weakness is apparent soon after birth with both the congenital and the neonatal forms of myasthenia and develops later in childhood with juvenile myasthenia (57). Botulism is an acute paralytic disorder caused by ingestion of neurotoxin released by Clostridium botulinum. The toxin is found in food that is contaminated with the organism and processed under anaerobic conditions (58). Patients who ingest the toxin manifest generalized muscle weakness within 36 hours. Infant botulism is a form of the disease unique to children under 9 months of age. The organism is ingested in vivo and colonizes the gastrointestinal tract of the infant, leading to a slow release of toxin. These patients most commonly present between 2 and 4 months of age and manifest poor feeding, constipation, lethargy, and generalized hypotonia. In severe cases, these infants may require emergent intubation and mechanical ventilation (59,60,61).
Common Clinical Scenarios
Cardiopulmonary Resuscitation
The most frequent cause of cardiac arrest in pediatric patients is a preceding respiratory arrest. In a review of the causes of cardiac arrest in 119 patients younger than 18 years of age, the most common presentation was SIDS (32%), followed by drowning (22%), other respiratory causes (9%), congenital cardiac problems (4%), cancer (3%), other cardiac causes (3%), drug overdose (3%), and smoke inhalation (2%) (62). Initial attempts to restore oxygenation and ventilation should first be performed with BVM ventilation. If no spontaneous respirations return, the practitioner should proceed with endotracheal intubation. During cardiopulmonary resuscitation, certain medications (atropine, epinephrine, lidocaine, and naloxone) may be delivered via the endotracheal tube and absorbed into the systemic circulation (63).
Airway Protection
Patients with a depressed mental status and absent cough and gag reflexes should undergo endotracheal intubation to prevent aspiration of oropharyngeal secretions and/or gastric contents. Potential causes for this type of presentation include drug ingestion, trauma, metabolic encephalopathy, and intracranial mass lesion. Intubation is particularly important for such patients if gastric lavage will be performed for a suspected ingestion.
Facilitating Hypocapnic Ventilation
Lowering the PaCO2 will decrease cerebral arterial blood flow and lower intracranial pressure. Patients suspected of having an intracranial mass lesion with impending brain herniation should undergo emergent endotracheal intubation to protect the airway and reduce intracranial pressure using hypocapnic ventilation before an imaging study is obtained. Performing a “neurologic induction” to minimize any rise in intracranial pressure during intubation may also be indicated in such cases (see Chapter 15).
Ventilatory Support during Circulatory Failure
The child with circulatory failure may have compromised oxygen delivery from both poor circulation and respiratory dysfunction. In addition, decreased respiratory pump function may result from diminished respiratory muscle perfusion, acidosis, hypoxia, and electrolyte abnormalities (64). In this situation, endotracheal intubation and mechanical ventilatory support may improve cardiac output and oxygen delivery while decreasing the work of breathing. Cautious use of sedatives and preparation for rapid administration of intravenous fluids should be established before attempting intubation of patients in circulatory failure because the procedure itself may precipitate hypotension.
Facilitating Tracheobronchial Suctioning
Patients with thick, tenacious secretions due to respiratory infection or mucociliary abnormalities (cystic fibrosis, Kartagener syndrome) may be unable to adequately clear airway secretions. Such patients will present with respiratory distress and hypoxemia. Tracheal intubation will provide access to the airway below the vocal cords, allowing suctioning with saline lavage and improved pulmonary toilet (see Chapter 79). Newborns delivered in the ED suspected of having significant meconium aspiration may also require intubation and tracheal suctioning (see Chapter 36).
Use of Specific Approaches
Adults are commonly intubated awake, either orally or nasally, when they have an increased risk for complications with the use of anesthetic agents or muscle relaxants. Awake intubations are normally performed after the application of topical anesthesia and administration of a sedative or analgesic. However, even with excellent topical anesthesia and sedation, it is usually difficult or impossible to talk a child through an awake procedure. In children less than 4 to 6 months of age, awake intubation is frequently used to avoid both the risks
associated with anesthetic agents and the loss of protective airway reflexes. Rarely does harm come from an attempt at awake intubation, and many authorities recommend this approach in appropriate circumstances. Indications for awake intubation include (a) known anatomic abnormality of the airway, (b) inexperience with pediatric intubation, (c) circulatory instability, and (d) severe hypoxemia. Even a short disruption in ventilation may significantly worsen pre-existing hypoxemia, and the hemodynamically unstable patient often will not tolerate the circulatory effects of sedatives or analgesics.
associated with anesthetic agents and the loss of protective airway reflexes. Rarely does harm come from an attempt at awake intubation, and many authorities recommend this approach in appropriate circumstances. Indications for awake intubation include (a) known anatomic abnormality of the airway, (b) inexperience with pediatric intubation, (c) circulatory instability, and (d) severe hypoxemia. Even a short disruption in ventilation may significantly worsen pre-existing hypoxemia, and the hemodynamically unstable patient often will not tolerate the circulatory effects of sedatives or analgesics.
By far the majority of emergent and urgent tracheal intubations in children are performed via the oral route using direct laryngoscopy. Blind nasotracheal intubation is rarely successful in patients younger than 8 years of age because (a) it requires a substantial degree of patient cooperation; (b) the larynx is in a more superior position; and (c) the vocal cords are angled, resulting in poor alignment of the nasopharyngeal airway and glottic opening. Additionally, children commonly have enlarged adenoids that may be traumatized during passage of a nasotracheal tube, often causing significant bleeding. Consequently, nasal intubation should not be performed for urgent or emergent airway control for young children, although this approach may be considered for older children or adolescents. As mentioned previously, several alternative techniques for endotracheal intubation have been developed for the “known difficult airway” and “failed airway” situations. These methods are described in Chapter 17.
Contraindications
No absolute contraindications exist for securing the airway. Because inadequate oxygenation rapidly leads to brain injury and death, control of the airway takes precedence over other considerations in the severely compromised patient. However, one circumstance does exist in which endotracheal intubation should not be the primary method of securing the airway—an unstable patient with blunt or penetrating injury to the larynx should undergo emergency cricothyrotomy or tracheotomy without attempted direct laryngoscopy. When the larynx is fractured or disrupted, an endotracheal tube passed through the vocal cords may dissect into the soft tissues of the neck, creating a traumatic false passage. When positive pressure ventilation is then attempted, gas will be forced into the soft tissues, further distorting the anatomic structures of the neck and making attempted tracheotomy difficult or impossible.
Situations occur in which endotracheal intubation should be delayed if possible until additional interventions can be performed, additional personnel are available (e.g., an anesthesiologist or otolaryngologist), and/or the patient can be moved to a more controlled setting (Table 16.5). The decision to delay intubation obviously depends on the degree of respiratory compromise and how rapidly the patient’s clinical condition is deteriorating. A problematic intubation should be anticipated whenever the patient has a prior history of either difficult intubation or episodes of airway obstruction that suggest an anatomic abnormality. Clearly, obtaining a detailed history in this regard may not be possible with a critically ill patient. However, a brief physical examination will often reveal findings that may make both manual ventilation and visualization of the larynx difficult (e.g., macroglossia, micrognathia, facial clefts, midface hypoplasia, facial asymmetry, small mouth, or short neck). Limited mobility of the temporomandibular joint or cervical spine also increases the difficulty of visualizing the larynx by direct laryngoscopy. Patients with head and neck trauma may have midfacial instability, airway bleeding, edema, masses, or foreign bodies that distort or obscure normal airway anatomy. The operator should carefully plan the approach to intubation for these patients, ideally having available the items necessary for at least one backup method as well as the standard intubation equipment (see Chapter 17). Whenever the patient’s clinical condition allows, it is prudent in these situations to delay intubation until the patient can be transferred to the ICU or operating room, where procedures such as fiberoptic bronchoscopy or emergent tracheostomy can be more easily performed if necessary.
TABLE 16.5 Situations in Which Increased Risk of Complications from Endotracheal Intubation Occur | |
|---|---|
|
Contraindications to awake intubation with a pediatric patient include raised intracranial pressure and an unstable cervical spine. As mentioned previously, elevation of intracranial pressure associated with laryngoscopy and intubation should be suppressed using appropriate medications for patients suspected of having a process such as intracranial hemorrhage, severe traumatic brain injury, or intracranial mass lesion (see Chapter 15). In addition, head and neck movement by a struggling patient with an unstable cervical spine can potentially exacerbate a cord injury. When endotracheal intubation is immediately necessary in this situation, many authorities recommend that the clinician (a) immobilize the neck with in-line stabilization, (b) perform a rapid sequence induction, and (c) intubate the patient using the method that is least likely to produce movement of the neck and that the operator is capable of performing.
In many cases, the likelihood of complications as a result of an endotracheal intubation may be minimized if, instead of being performed immediately, the procedure is momentarily delayed until after intravenous access is obtained and medications are given or other therapy is implemented. For
example, most patients who require emergent intubation are at increased risk for aspiration of gastric contents. When time allows, intravenous administration of the medications used for rapid sequence induction can often significantly diminish this risk. Patients with pre-existing hypovolemia or shock should ideally receive a bolus of intravenous fluid before intubation, because positive pressure ventilation can exacerbate hypotension. It is important to remember that these are only relative contraindications to immediate intubation. Securing control of the airway is always the first priority, and the operator must not wait until fluids or medications can be administered if the patient is rapidly deteriorating because of inadequate oxygenation. However, when the airway can be appropriately managed on a temporary basis using manual ventilation, such interventions can significantly reduce the incidence of morbidity associated with emergent intubation.
example, most patients who require emergent intubation are at increased risk for aspiration of gastric contents. When time allows, intravenous administration of the medications used for rapid sequence induction can often significantly diminish this risk. Patients with pre-existing hypovolemia or shock should ideally receive a bolus of intravenous fluid before intubation, because positive pressure ventilation can exacerbate hypotension. It is important to remember that these are only relative contraindications to immediate intubation. Securing control of the airway is always the first priority, and the operator must not wait until fluids or medications can be administered if the patient is rapidly deteriorating because of inadequate oxygenation. However, when the airway can be appropriately managed on a temporary basis using manual ventilation, such interventions can significantly reduce the incidence of morbidity associated with emergent intubation.
Equipment
An important goal that must be accomplished before endotracheal intubation is to ensure that all necessary equipment is readily available. Reaching a crucial step only to find that a needed piece of equipment is not at hand can force the operator to abort the procedure and start over from the beginning. In many instances, emergent intubation must be performed with little warning or time to prepare. Therefore, it is highly important that all equipment necessary for intubation be assembled and checked before a patient with respiratory failure arrives in the ED. Preparing a cart that contains all the items needed to intubate any patient from neonate to adult is one method of organizing the necessary materials. One drawer may contain all the endotracheal tubes, ranging in size from 2.5- to cuffed 8.0-mm tubes. Another drawer can be used to hold an array of laryngoscope blades and extra laryngoscope handles. A third drawer may contain useful adjuncts for BVM ventilation and endotracheal intubation, such as Yankauer and flexible suction catheters, stylets of different sizes, oral airways from size 50 to 100 mm, nasopharyngeal airways, tincture of benzoin, and tape. A lockable drawer can hold syringes and medications. Other such systems can be devised based on the needs of each individual facility. Whatever method is used, adequate stocking with functional equipment should be checked at each shift, and materials must be cleaned and replaced immediately after they are used. A helpful memory aid in going through the mental checklist of equipment needed for an endotracheal intubation is SOAPIM (a variation on the “SOAP” mnemonic)—suction, oxygen, airway equipment, pharmacologic agents, intravenous access, and monitors (Table 16.6).
Suction
A large-bore (14 French) flexible suction catheter is preferred when intubating children under 1 year of age. The catheter should be multi-orifice and without a control port (i.e., it should provide continuous suction). These catheters are easily directed in the mouth of an infant and are less cumbersome than a Yankauer-style suction device when mouth opening is limited. The vacuum source should always be on full (200 cm H2O). For older children who consume solid foods, a Yankauer suction device is superior because of its large-diameter suction orifice and rigid design, allowing the operator greater ease in removing particulate matter (see also Chapter 13).
TABLE 16.6 Equipment Checklist: Soapim Mnemonic | |
|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree