Water loss
Unreplaced loss of solute-free water raises the serum sodium concentration and is the most common cause of hypernatremia. Concurrent sodium loss may occur, but with water loss in excess of sodium loss.
Major causes:
Gastrointestinal (diarrhea, vomiting, or both with inadequate fluid intake)
Insensible water loss (fevers, exposure to heat, exercise, infants with poor feeding)
Urinary water loss (central or nephrogenic diabetes insipidus, renal disease with urinary concentrating defect, therapy with loop diuretics)
Impaired thirst/hypodipsia (hypothalamic lesions, holoprosencephaly, osmoreceptor injury leading to essential hypernatremia)
Osmotic diuresis (glucose, urea, mannitol) in which urine is hypotonic to plasma due to nonreabsorbed osmotically active solute
Excess sodium
Exogenous sodium intake
Commonly iatrogenic (oral or intravenous sodium chloride or sodium bicarbonate)
Salt poisoning in infants and toddlers
Sodium retention
Mineralocorticoid excess can result in mild hypernatremia with reset osmostat
Intracellular water movement
Occurs during seizures or exercise due to transient intracellular increase in osmotically active molecules (lactic acid)
TABLE 32-1 Normal Serum Pediatric Laboratory Values (found at http://www.pediatriccareonline.org) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Variable and nonspecific
Depend on the magnitude of hypernatremia and the rate of change
Irritability, lethargy, weakness, MS changes, increased deep tendon reflexes, seizures, and cramping
Water loss can be accompanied by dehydration, volume depletion, and weight loss
Sodium excess can have weight gain, mild volume expansion, and edema
Assess weight change if possible. Weight loss may occur even in the absence of ECF volume depletion
Assess adequacy of ECF compartments
Intravascular compartment: peripheral pulses, perfusion, temperature, and capillary refill time
Interstitial compartment: skin turgor, tears, and mucous membrane appearance
Basic metabolic panel
Urinalysis and urine osmolality, sodium, and creatinine
TABLE 32-2 Assessment of Hypernatremia | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Normal saline to restore ECV. Initial bolus of 20 mL/kg over about 30 minutes in children without known underlying cardiac or renal disease.
Close monitoring and reassessment is critical.
Reduce the serum sodium level slowly (approximately 0.5 mEq/L/hour and no greater than 15 mEq/L in a 24-hour period).
Assessment of the solute-free water deficit, maintenance needs, and ongoing losses of water ± sodium should be performed. The free water deficit is calculated as follows:
4 mL/kg of free water decreases serum Na+ by 1 mEq/L
The free water deficit (L)= 4 mL/kg× wt(kg)× (observed Na+– desired Na+)
Hypotonic intravenous fluids at a concentration and rate determined by careful calculation and given over 48 hours is recommended.
Avoid rapid administration of very hypotonic fluids, which can lead to intracellular water shift, cell swelling, and cerebral edema.
Monitor serum sodium, fluid balance, and weight closely with any intravenous fluid therapy.
Patients with severe total body sodium excess require judicious use of fluids, with or without furosemide administration, dialysis, or both (under the guidance of a nephrologist).
Dilutional hyponatremia. The total body water is generally expanded and is increased relative to total body sodium.
Depletional hyponatremia. There is a decreased (or normal) amount of total body water and a deficit of sodium in excess of water.
Pseudohyponatremia. Sodium is not distributed throughout the serum and its concentration is artificially lowered. Causes of pseudohyponatremia include hyperlipidemia and hyperproteinemia. Na+ decrease = 0.002 × lipid concentration in mg/dL (hyperlipidemia) and 0.25 × (protein in g/dL -8) (hyperproteinemia).
Severe hyperglycemia. Hyponatremia may occur from osmotic movement of water out of cells. Na+ decreases by 1.6 mEq/L per 100 mg/dL rise in glucose.
TABLE 32-3 Causes of Hyponatremia | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Variable and nonspecific
Depend on the magnitude of hyponatremia and the rate of change
Lethargy, weakness, encephalopathy, and seizures
Assess weight change and status of ECF volume as with hypernatremia.
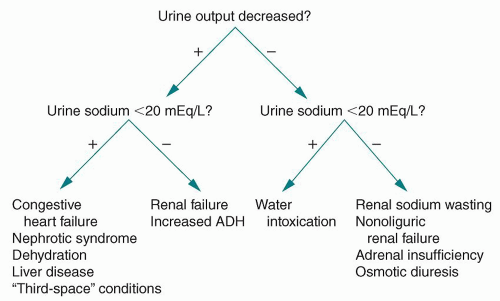 FIGURE 32-1 Narrowing the differential diagnosis for the underlying cause of hyponatremia. ADH, antidiuretic hormone. |
Basic metabolic panel
Serum osmolality: Estimated by doubling the serum sodium level (mEq/L) and adding 10 or the following equation:
Serum osm = 2 × Na+ (mEq/L) + Glucose/118 + BUN/2.8
Urine osmolality
Urine sodium
Serum and urine uric acid (helpful in SIADH)
Calculation of the fractional excretion of sodium (FE Na) can be useful when the urine sodium concentration is borderline (e.g., 19 to 21 mEq/L):
FENa = (UNa × PCr)/(UCr × PNa) × 100%
Compromised ECV. Restore promptly with the administration of isotonic saline, initially with an intravenous bolus of 20 mL/kg, given rapidly over about 30 minutes. Additional isotonic fluid boluses may be indicated after patient reassessment.
Sodium deficit. The sodium deficit should be calculated and measures taken to restore the sodium level. The sodium deficit can be calculated as follows:
Na+ deficit (mEq) = 0.6 × wt (kg) × (desired Na+ – observed Na+)
Rapid increase in serum sodium with 3% saline is required in symptomatic patients (e.g., hyponatremia-induced seizures) 0.5 mEq/mL.
Volume of 3% (mEq) = (target Na+ – observed Na+) × wt (kg) × fD (fD – distribution factor, for Na+ 0.6-0.7 L/kg body weight).
In acute hyponatremia, the sodium level should be corrected to 125 mEq/L or to a level slightly higher than that associated with symptoms.
In chronic hyponatremia, rapid correction can lead to osmotic demyelinization syndrome or central pontine myelinolysis.
Target rate of Na+ rise in asymptomatic patients: 0.5-1 mEq/L/hour or 10-20 mEq/24 hour.
General principle of treatment is restriction of fluid.
Exceptions include edematous states with hypoalbuminemia, where there is risk of intravascular volume depletion.
Restrict sodium, as it may contribute to worsening of associated edema.
SIADH: Treatment approach
Uncommon in pediatrics, usually transient. See Table 32-3 for medications causing SIADH.
ADH is fixed regardless of variation in intake, ECF volume, or serum osmolality.
Fluid restriction to approximately 2/3 of maintenance.
 HINT: Serum sodium concentration is maintained within a narrow range by adjustments in water intake and excretion in response to ADH secretion and thirst. Abnormalities in serum sodium are therefore predominantly a result of abnormalities in water balance, with or without a significant contribution from sodium itself; however, sodium may be a predominant factor in certain specific sodium derangements.
HINT: Serum sodium concentration is maintained within a narrow range by adjustments in water intake and excretion in response to ADH secretion and thirst. Abnormalities in serum sodium are therefore predominantly a result of abnormalities in water balance, with or without a significant contribution from sodium itself; however, sodium may be a predominant factor in certain specific sodium derangements.
Transcellular potassium shifts are the most common cause of hyperkalemia in childhood. Potassium shifts from the ICF to the ECF in the following conditions:
Metabolic acidosis
Beta-adrenergic blockade
Strenuous exercise
Insulin deficiency, hyperglycemia, and hyperosmolality
Hyperkalemic periodic paralysis
Increased potassium intake
Potassium supplements, drugs containing potassium (e.g., potassium penicillins), salt substitute use, and blood transfusions (stored blood). Hyperkalemia usually occurs in the context of impaired renal potassium excretion.
Decreased renal excretion of potassium
Renal failure
Medications (Table 32-4)
Renal tubular acidosis (RTA) – Type IV or voltage-dependent distal (Type I) RTA
Mineralocorticoid deficiency (adrenal insufficiency, congenital adrenal hyperplasia (CAH), hyporeninemic hypoaldosteronism, and primary mineralocorticoid deficiency).
Mineralocorticoid resistance (transient condition in newborn, associated with renal injury, or due to pseudohypoaldosteronism).
Increased endogenous cellular release of potassium is associated with hypoxic or toxic cell death, burns, intravascular hemolysis, rhabdomyolysis, and acute tumor lysis syndrome.
Pseudohyperkalemia – Movement of potassium out of cells during or after specimen collection.
Hemolysis
Leukocytosis or thrombocytosis
Familial pseudohyperkalemia (temperature-dependent leakage of potassium out of cells)
TABLE 32-4 Drugs Associated with Hyperkalemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Serum electrolyte panel; serum calcium, magnesium, and glucose
Urinalysis, urine potassium, and creatinine (to calculate the transtubular potassium gradient or TTKG)
Other tests (e.g., imaging, endocrine, or genetic studies) based upon clinical suspicion
ECG
Peaked T waves (especially in precordial leads) and prolonged PR intervals or a widened, prolonged QRS complex
Late life-threatening ECG changes include flattened P or T waves (or both) with ST segment depression, a sine wave pattern, and tachyarrhythmias or bradyarrhythmias
A potassium value above 7.0 mEq/L
A rapidly increasing potassium concentration
A clinical state in which the potassium level is expected to continue to increase (e.g., rhabdomyolysis)
Renal failure
Symptoms of hyperkalemia
 HINT: Avoidance of hyperkalemia is critical in patients with kidney disease and reduced glomerular filtration rate (GFR). These patients require frequent lab monitoring. Some may require restriction of potassium intake, avoidance of certain medications (e.g., renin-angiotensin system blockade), and use of potassium-free solutions for intravenous fluid therapy. All patients receiving intravenous fluids with potassium should have renal function assessed prior to administration.
HINT: Avoidance of hyperkalemia is critical in patients with kidney disease and reduced glomerular filtration rate (GFR). These patients require frequent lab monitoring. Some may require restriction of potassium intake, avoidance of certain medications (e.g., renin-angiotensin system blockade), and use of potassium-free solutions for intravenous fluid therapy. All patients receiving intravenous fluids with potassium should have renal function assessed prior to administration.
Severely limited nutrition (e.g., anorexia nervosa, renal excretion can adjust to more moderate limitations in potassium intake)
Increased gastrointestinal losses (e.g., vomiting, diarrhea, cathartic abuse)
Increased skin losses (e.g., excessive sweating, burns)
Increased renal losses— Fanconi syndrome, RTA, Bartter syndrome, diuretic therapy, osmotic diuresis (e.g., glucosuria), hyperaldosteronism, CAH, 11-b-hydroxysteroid dehydrogenase deficiency (or inhibition with natural licorice ingestion), Liddle syndrome, Gitelman syndrome (magnesium-losing tubulopathy), salt-wasting nephropathies, excess adrenocorticotropic hormone (ACTH), drugs (Table 32-6), magnesium depletion, chloride depletion, loss of gastric secretions, and polyuria
Transcellular potassium shifts— alkalosis (metabolic and respiratory), excess insulin, beta-adrenergic activity, hypokalemic periodic paralysis, and certain drugs (Table 32-6)
TABLE 32-5 Treatment of Hyperkalemia | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



