SERM
Breast
Uterus
Tamoxifen
−
++
Raloxifene
−
−
Toremifene
−
+/−
Idoxifene
−
+/−
Droloxifene
−
+/−
GW 5638
−
+/−
Arzoxifene
−
−
EM-652
−
+/−
Many of the epidemiologic and genetic risks which predispose to breast cancer can also increase the risk of endometrial cancer, and the women taking tamoxifen for 5 years have two- to threefold increased risk for developing endometrial cancer than the age-matched population [6–8]. Use of 10 years would produce an additional risk of about 2 % by 15 years [3]. The cumulative risk and mortality from endometrial cancer also increase with 10 years of tamoxifen with an absolute mortality increase of 0.2 % [1]. The risk of endometrial cancer is greatly outweighed by the reduction in breast cancer mortality. Raloxifene, a second-generation SERM, seems to prevent invasive breast cancer but with only 76 % effect of tamoxifen in high-risk women and has few life-threatening side effects including endometrial cancer [9]. Cochrane database systematic review 2012 showed that toremifene and tamoxifen are equally effective in breast cancer treatment and safety profile of toremifene is not worse than tamoxifen.
Endometrial Abnormalities Associated with Tamoxifen
The estrogenic effect of tamoxifen on endometrium causes endometrial hyperplasia, polyp, endometrial cancer, and uterine sarcoma [10, 11]. Study by Cheng et al. found no significant endometrial abnormalities in premenopausal patient treated with tamoxifen whereas postmenopausal patients on tamoxifen had significant endometrial abnormalities [12].
The association between endometrial abnormalities and dose of tamoxifen had been addressed in several studies. The incidence of endometrial abnormalities in postmenopausal patients on tamoxifen is reported to be around 40 %. Development of endometrial cancer is time and dose dependant. Prolonged tamoxifen intake more than 35 g seems to be associated with increased risk of endometrial cancer, and a substantial number is constituted by type II endometrial cancer. These are found to be p53 positive and ERα, PRα, and PR β negative [13]. Tamoxifen also has been proposed to interact with ERα in endometrium to induce insulin-like growth factor I (IGF-I) signaling via IGF-I R, thereby activating P13K/AKT/mToR pathway. This protein expression may lead on to development of targeted therapies in this group of patients [14].
The most common endometrial abnormality associated with tamoxifen is endometrial polyp (Fig. 41.1). These polyps are usually large and characterized by combination of cystically dilated glands, metaplasia, and stromal decidualization. The extensive stromal fibrosis causes difficulty in hysteroscopic resection. Malignant changes were seen in 3–7 % of these polyps. The reported incidence of endometrial hyperplasia in patients on tamoxifen is mainly from sonographic findings, and there is discordance between endometrial thickness and histopathology. Recurrent endometrial polyps are reported in association with tamoxifen exposure [15].
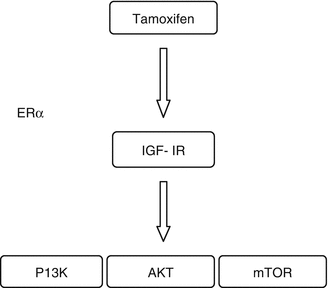

Fig. 41.1
Insulin-like growth factor signaling in endometrial carcinoma exposed to tamoxifen
Histopathological review of endometrium in 700 patients who had 20 mg tamoxifen for mean of 4 years showed 4.7 % endometrial carcinoma, 8 % hyperplasia, 12.9 % polyps with atypical hyperplasia, and 23% benign polyp [16]. Tamoxifen may exert variable effects at different parts of endometrium. An individual patient can have atrophic, hyperplastic, and malignant areas in the endometrium. Even though office endometrial biopsy is the diagnostic tool for endometrial hyperplasia, false-negative rate may be high in tamoxifen patients because of heterogeneous changes in the endometrium. This leads to the argument for hysteroscopy or dilatation and curettage for these patients. Endometrial polyps associated with tamoxifen are usually translucent edematous, and more fibrotic with metaplastic changes of epithelial component. The incidence of endometrial pathology is higher in symptomatic patients than asymptomatic patients.
Most of the literature regarding side effects of tamoxifen is from Western countries. But there are limited data regarding safety and adverse effect profile in Indian population. Tamoxifen seems to have less carcinogenic potential in eastern Indian women. In a report from Chittaranjan National Cancer Institute, Kolkatta, none of the 3000 patients that received tamoxifen developed endometrial cancer [17]. A retrospective analysis of 65 breast cancer patients with tamoxifen with endometrial histology from Regional Cancer Centre, Thiruvananthapuram, showed 7 endometrial polyps, 5 atypical hyperplasias, 3 simple hyperplasias, and 8 endometrial malignancies including two carcinosarcomas (unpublished data). These patients were either symptomatic with abnormal vaginal bleeding or had sonography detected thickened endometrium. Of the 8 patients who had endometrial malignancy, 5 (62.5 %) patients had tamoxifen exposure of more than 48 months and seven were symptomatic with abnormal vaginal bleeding (unpublished data).
Gynecological Surveillance
Gynecologic surveillance in patients on tamoxifen still remains an area of concern among physicians in spite of various recommendations. Since the early 1990s various case series and reports have been published on the role of transvaginal ultrasound and endometrial sampling as screening to reduce the incidence of endometrial carcinoma. Two prospective randomized studies concluded that endometrial biopsy or transvaginal ultrasound is not warranted in patients on tamoxifen who are asymptomatic [18, 19]. The study by Barakat et al. [18] included 111 pre- and postmenopausal patients on tamoxifen with median age of 50 years. They underwent a total of 635 endometrial biopsies with an average of 5.8 per patient. 86 % of the samples were benign and 12 % were insufficient sampling which was considered as atrophic endometrium. Unnecessary surgical interventions and the costs associated are not justified when compared to the incidence or prognosis of tamoxifen-associated endometrial cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


