Background
Preterm delivery is a leading cause of neonatal morbidity and death. It often results from chorioamnionitis, which is a complication of bacterial vaginosis. Probiotics are effective in the treatment of bacterial vaginosis in women who were not pregnant; studies in pregnant woman are missing.
Objective
The purpose of this study was to evaluate whether an oral probiotic food supplement supports the maintenance or restoration of a normal vaginal microbiota during pregnancy.
Study Design
We conducted a randomized, placebo-controlled, triple-blind, parallel group trial. Oral Lactobacillus rhamnosus GR-1and L reuteri RC-14 (10 9 colony-forming units) or placebo were administered for 8 weeks to women with <12 completed weeks of pregnancy. Participants were enrolled at Tuebingen University Hospital and 10 recruiting gynecologic practices. Vaginal swabs were taken before and after intervention and analyzed according to the Nugent scoring system. Telephone interviews were performed before and after intervention and after delivery. Primary outcome was the proportion of swabs with normal Nugent score (<4) after intervention, compared by Fisher’s exact test in an intention-to-treat analysis.
Results
Three hundred twenty pregnant women were enrolled. Vaginal swabs were analyzed from 290 women before and 271 women after intervention. The proportion of normal vaginal microbiota decreased from 82.6 to 77.8% in the treatment group and from 79.1 to 74.3% in the placebo group, with no significant difference across groups after intervention ( P =.297).
Conclusion
Oral probiotics may be suitable for implementation in antenatal care but, as administered here, had no effect on vaginal health during mid gestation. Other application routes or probiotic preparations may be more effective in supporting vaginal microbiota during pregnancy.
P reterm delivery (PD) is defined as birth at <37 weeks of pregnancy (WOP). In Germany, PD affects approximately 9% of pregnancies, which ranks Germany seventh highest among 39 highly developed countries worldwide. Each year, approximately 60,000 infants are born preterm in Germany. PD accounts for 75% of perinatal deaths and more than one-half of long-term morbidity. It is the leading cause of death in the neonatal period. Approximately 30% of preterm births are indicated for maternal (preeclampsia or eclampsia) or fetal reasons (growth retardation, placental insufficiency). However, more than two-thirds occur as spontaneous preterm births, either after spontaneous preterm labor (40%) or premature preterm rupture of membranes (30%). Despite all efforts to refine postnatal care, the best way to improve outcome of these newborn infants is to prevent PD.
A common pathway to the inducement of preterm labor, which in turn leads to PD, is chorioamnionitis, which results from subclinical ascending infection, which is associated with changes in the vaginal microbiota. Determinants that lead to an abnormal vaginal microbiota with changes in lactobacilli composition are unknown but may be relevant for innovative prognostic and therapeutic approaches. In most cases, the lactobacilli-dominated microbiota is overgrown by potentially pathogenic rods that ascend from the gut, which are called bacterial vaginosis (BV). BV was shown repeatedly to be an important and independent risk factor for PD. Treating BV with antibiotics, however, although it resolved the symptoms of BV, did not prevent PD. Assuming that the course of events that ultimately results in PD starts well before the manifestation of BV can be diagnosed, early screening for changes in vaginal pH has been suggested but showed no consistent effect on PD rates. So far, studies that have aimed to show efficacy of probiotics in the prevention of PD have been underpowered.
Oral intake of Lactobacilli-composed probiotics has been shown to reduce pathogenic bacteria in the vaginal microbiota of women who were not pregnant. Data on its effect on the vaginal microbiota of pregnant women are missing. Hence, in this study and with the ultimate goal of the prevention of PD, we investigated the effect of a commercially available food supplement that consisted of 2 probiotic Lactobacilli strains ( Lactobacillus rhamnosus GR-1 and L reuteri RC-14) on the vaginal microbiota of pregnant women. We hypothesized that these probiotics strains support the maintenance and/or restoration of a normal vaginal microbiota in pregnant women.
Methods
Ethics statement
The Ethics Committee of Tuebingen University Hospital approved the study protocol.
Study design
Trial design
A randomized, placebo-controlled, triple-blind, parallel group trial was designed (trial number ISRCTN40042090).
Participants
The study population comprised pregnant women who lived within the study area at the time of enrolment and who were attached to 1 of 10 recruiting obstetric practices. A random selection process assured representativeness of recruiting practices, hence, external validity. Inclusion criteria for participants were age >18 years, less than 12 completed WOP, and informed written consent. Women with active chronic inflammatory bowel disease (Morbus Crohn, colitis ulcerosa) or immunodeficiency were excluded. There were no other exclusion criteria such as common intake of other probiotics or intake of antibiotics before intervention. Enrolment occurred between October 2010 and May 2012. Pregnant women were approached and informed about the study by their gynecologist during a routine antenatal care visit. Final recruitment took place through a phone call from the study center at the Department of Neonatology, Tuebingen University Children’s Hospital. Only women who had given their written informed consent were included in the study. Before a practice began to recruit patients, a prestudy initiation visit was performed to ensure availability and completeness of all study materials and adequate teaching of the recruiting gynecologists. Reports of prestudy visits were provided to the investigators.
Interventions
After enrolment, women were assigned randomly to treatment or placebo group, and study treatment was delivered via standard mail. Participants were instructed to begin with study treatment only after their next antenatal care visit. A first questionnaire-based telephone interview was conducted that covered demographic and clinical variables and information about participants’ adherence to the study protocol. During the first routine antenatal care visit after enrolment and randomization, preintervention vaginal swabs were taken with CultureSwab Plus collection tubes (Becton Dickinson, Heidelberg, Germany). Participants were instructed again about the study protocol and the beginning of the intervention period, which started on the day of this routine antenatal care visit. Intervention consisted of daily oral intake of 1 capsule that contained L rhamnosus GR-1 and L reuteri RC-14 (1×10 9 colony-forming units of each strain per capsule) or placebo that contained lactose (Christian Hansen, Hørsholm, Denmark) for 8 weeks. The quality of the product was controlled regularly by the manufacturer and verified for the study medication lot. Shortly after the end of the intervention period, a second telephone interview was conducted that covered the participants’ adherence to the study protocol and the occurrence of any side-effects. A second vaginal swab was taken during the first routine antenatal care visit after intervention. Swabs were returned immediately to the Institute of Medical Microbiology at Tuebingen University Hospital for analysis. Gram-staining was performed according to standard methods. Nugent score was determined according to Nugent et al. Scores 0–3 were classified as normal microbiota; scores 4–6 were classified as intermediate microbiota, and scores 7–10 were classified as BV. For further analysis, intermediate microbiota and BV were subsumed as abnormal microbiota (see Statistical Methods). Shortly after their due date, participants were again contacted by the study center to complete a short telephone questionnaire concerning the course and duration of pregnancy and any potential side-effects.
Outcomes
The primary outcome was the proportion of normal vaginal microbiota defined as Nugent score <4 after 8 weeks of intervention. Secondary outcomes were Nugent score distribution, intervention-associated discomfort that did not lead to an interruption of study medication, defined pruritus or increased vaginal discharge, abdominal discomfort, changes in stool consistency, duration of pregnancy, proportion of preterm deliveries (ie, between 22+0 and 36+6 WOP), miscarriages (delivery before 22+0 WOP), and pregnancy duration.
Sample size
Power calculation was based on a randomized placebo-controlled trial in healthy women who were not pregnant who were treated orally with L rhamnosus GR-1 and L reuteri RC-14 for 60 days and showed an increase in the proportion of women with normal vaginal microbiota (ie, Nugent score <4) from 28–42% when assessed in vaginal swabs on day 90 and a significant increase in lactobacilli and decrease in pathogenic bacteria. In this study, we aimed to detect an increase in normal vaginal microbiota by (at least) 50%. Assuming a 25% baseline risk for abnormal vaginal microbiota after intervention in our sample, an allocation ratio of 1:1, 1-tailed Fisher’s exact test as the preferred analysis, a type-I-error of 0.05 and a type-II-error of 0.2 (ie, a power of 80%), the calculated total sample size was 320 pregnant women.
Interim analysis and stopping guidelines
Animal and clinical studies suggest that the probiotic strains that were used in this trial are safe during pregnancy. Therefore, the study did not include any stopping rules, neither for participants nor for the total study.
Randomization sequence generation, type and implementation
Randomization was performed with the use of a computer-generated random number list. Subjects were allocated to study arms with the use of simple block randomization.
Blinding
Recruiting gynecologists and study center (administering treatment), subjects, and study center personnel including the analyzing microbiologist (evaluating the response to treatment) were all blinded to group assignment until study completion and final analyzes (ie, triple-blind design). Only the study statistician and the employee of the University Hospital’s pharmacy were aware of group assignment, thereby ensuring concealment of study group allocation. The employee of the chemist’s shop allocated subjects according to the computer-generated random number list.
Similarity of intervention
The placebo was matched to the study drug for taste, color, and size and was not distinguishable from treatment, neither by visual inspection nor by taste.
Statistical methods
Demographic and clinical characteristics of participants were analyzed with the use of descriptive statistics such as number and percentage or median, minimum and maximum. Nugent score as the primary outcome was dichotomized in normal (score, 0–3) and abnormal (ie, intermediate/pathologic microbiota; score, 4–10). Frequencies were compared after the intervention with the use of Fisher’s exact test. For this comparison, a probability value of <.05 was regarded as statistically significant. Risk ratios and 95% confidence intervals were calculated by Cochrane-Mantel-Haenszel-statistics with the use of SAS software (SAS Institute, Heidelberg, Germany), which accounted for competing risks. For all secondary outcomes presented, probability values have only descriptive character. This was done using SPSS (version 20; IBM Inc., Ehingen, Germany).
Results
Of 320 women who were assigned randomly, 144 pre- and 135 postintervention samples in the treatment vs 148 pre- and 136 postintervention samples in the placebo group could be analyzed. The main reasons that led to missing samples were spontaneous abortions and logistic problems. The postintervention telephone questionnaires were completed by >90% of subjects in either group ( Figure ).
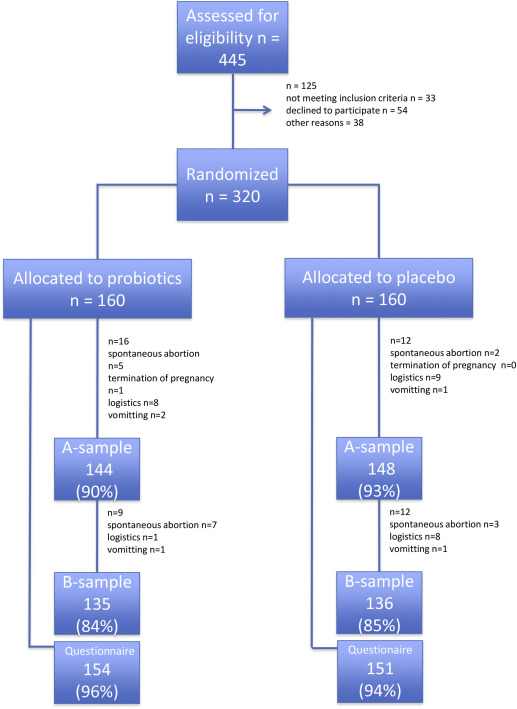
Parameters that potentially influenced the baseline risk of aberrant vaginal microbiota and PD (such as maternal age, multiparity, history of urogenital infections, or a history of PD) and the use of antibiotics before the intervention period were distributed equally between groups. Compliance with taking the study medication and data concerning the use of other probiotics also did not differ between groups ( Table 1 ).
| Characteristic | Total (n=320) | Treatment (n=160) | Placebo (n=160) |
|---|---|---|---|
| Median maternal age, y (range) | 33 (21-45) | 33 (21-45) | 33 (24-44) |
| Married, n (%) | 286 (90) | 141 (88) | 145 (91) |
| Primiparous, n (%) | 122 (38) | 61 (38) | 61 (38) |
| Multiple pregnancy, n (%) | 15 (5) | 10 (6) | 5 (3) |
| History of preterm delivery, n (%) | 23 (7) | 13 (8) | 10 (6) |
| History of recurrent vaginal infections, n (%) | 50 (16) | 21 (13) | 29 (18) |
| Uterine malformation/operative procedures, n (%) | 35 (11) | 19 (12) | 16 (10) |
| Probiotics before pregnancy, n (%) | 56 (18) | 29 (18) | 27 (17) |
| Antibiotics 4 weeks before intervention, n (%) | 13 (4) | 5 (3) | 8 (5) |
| Weeks of pregnancy at start of intervention, wk+d | 12+2 | 12+1 | 12+2 |
| Compliance of capsule intake, n (%) | 275 (86) | 133 (83) | 142 (89) |
Contrary to our hypothesis, in the treatment group, the proportion of normal microbiota decreased from 82.6–77.8%, which is a decrease by 4.8 percentage points. The proportion of normal microbiota in the placebo group decreased from 79.1% before intervention to 74.3% after intervention, which is a decrease also by 4.8 percentage points. Comparing postintervention samples of treatment and placebo group, there was no significant difference in the occurrence of abnormal microbiota ( P =.297).
Overall, the BV rate was low in the study population, only 2.8–5.4% of participants had BV before intervention ( Table 2 ). After intervention, BV was present in 1.8% of women in the placebo group vs 2.2% in the treatment group.
| Nugent score | Treatment, n (%) | Placebo, n (%) | P value | |
|---|---|---|---|---|
| 1-Tailed | 2-Tailed | |||
| Normal microbiota sample | ||||
| A | 119 (82.6) | 117 (79.1) | .265 | .460 |
| B | 105 (77.8) | 101 (74.3) | .297 | .560 |
| Bacterial vaginosis sample | ||||
| A | 4 (2.8) | 8 (5.4) | .202 | .376 |
| B | 3 (2.2) | 2 (1.5) | .497 | 1.000 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


