Fig. 20.1
Diagrammatic/Schematic representation of May-Thurner syndrome (Courtesy (http://emedicine.medscape.com/article/2056380) [34])
Pregnant women are many a times advised bed rest and hence decreased mobility [46, 62]. They also have a tendency to be dehydrated due to excessive vomiting, sweating, etc. This leads to an increase viscosity of blood and the formation of microthrombi inside veins, which are not washed away by the sluggish venous return in pregnancy. The thrombus thus formed may grow and propagate.
Pregnancy is a hypercoagulable state [15]. This is due to the physiological changes in pregnancy. These changes are in both coagulation and fibrinolytic systems. There is an increase in endogenous thrombin generation, combined with a decrease in circulating plasma antithrombin and fibrinolysins. Normal pregnancy-associated changes in coagulation are increased concentrations of factors VII, VIII, and X and von Willebrand factor and marked increases in fibrinogen. Factors II, V, and IX are relatively unchanged [15]. The only blood coagulation factor that is decreased in pregnancy is factor XI. There is acquired resistance to activated protein C (an endogenous anticoagulant). Free protein S, which is its active and unbound form, is decreased during pregnancy. Plasminogen activator inhibitor type 1 (PAI-1) levels are increased almost five times in pregnancy. Toward the third trimester, levels of PAI-2 produced by the placenta increase drastically [82]. Prothrombin fragments (PF1 + 2), which are the markers of thrombin generation and thrombin–antithrombin (TAT) complexes, are also increased in pregnancy. These changes start to increase at conception and may take up to 8 weeks postpartum to return to baseline [20].
Risk Factors Associated with an Increased Risk for Thromboembolism
Age >35
Infection
Malignancy
Obesity
Personal/family history of thrombosis
Smoking
Surgery
Varicose veins
Blood loss >1 L
Cesarean birth
Forceps (midcavity or rotational)
Hyperemesis/dehydration
Multiparity >3
Multiple pregnancy
Preeclampsia
Pregnancy/puerperium
Prolonged labor >24 h
Cardiac disease
Nephrotic syndrome
Ovarian hyperstimulation syndrome
Paraplegia/pelvic trauma/long-distance travel
Prolonged immobilization
Sickle cell disease
Thrombophilia
This hypercoagulable state is indeed a protection against hemorrhage during abortion and delivery. Hemorrhage still accounts for the majority of maternal deaths in India. Endothelial (intimal) damage in the blood vessel may be intrinsic or secondary to external trauma. It may be from accidental injury or surgical insult.
Natural Inhibitors of Coagulation during Pregnancy
Antithrombin (AT) is a serine proteinase inhibitor which acts by interaction with its cofactor heparin [89, 109]. Thrombin is inactivated by AT either directly or by inactivating factors IX, X and XI by forming a covalent complex. These factors are inhibited very slowly. This process can be accelerated by the binding of heparin and heparin-like compounds to AT. Protein C gets activated by thrombin. The process is enhanced by the interaction of thrombin with thrombomodulin [26]. Activated protein C inactivates factors Va and VIIIa on the platelet and endothelial cell surface and hence serves to block thrombin generation. Protein C requires protein S, which is another vitamin K-dependent molecule as a cofactor. The imbalance between reduced inhibitors of coagulation and/or increased activation of coagulation factors can lead to thrombosis in pregnancy [20, 26, 114].
Fibrinolysis
Enzymes like plasmin are involved in the removal of thrombus from the circulation. The main role of plasmin is to degrade fibrin. Plasmin is present in its inactive form as plasminogen. The activation of plasminogen is by serine enzymes known as tissue-type plasminogen activator (t-PA) and urokinase (u-PA) [14]. This activity is regulated by specific inhibitors, plasminogen activator inhibitor (PAI)-1 and PAI-2. Plasminogen deficiencies can also lead to thrombophilia in patients.
Specific Types of Inherited Thrombophilias
The list of inherited thrombophilias well studied [4, 12, 17, 27, 31–33, 37, 41, 43, 45, 48, 60, 63–65, 67, 68, 73, 75, 78, 81, 83, 87, 88, 92, 95, 102, 103, 119, 120, 129] includes established genetic factors such as factor V Leiden mutation, prothrombin gene mutation; protein C, protein S, and antithrombin deficiencies; activated protein C resistance; prothrombin gene mutation; rare genetic factors like dysfibrinogenemia and hyperhomocysteinemia; elevated factors VIII, IX, and XI; elevated lipoprotein a; platelet glycoprotein gene polymorphisms; plasminogen deficiency; tissue plasminogen activator (tPA); plasminogen activator inhibitor (PAI); thrombomodulin gene defect; heparin cofactor II; and histidine-rich glycoprotein deficiency.
The lifetime probability of developing thrombosis in individuals with inherited thrombophilias compared to those with no defect shows that the highest incidence occurred with carriers of protein S deficiency, next with antithrombin deficiency, followed by protein C deficiency, and the least for factor V Leiden mutation. We have advanced a lot in our knowledge of congenital defects that predispose to thrombosis. This has made us understand the disease process of inherited thrombophilias as well as helped us recommend appropriate screening, detection, diagnosis, and treatment of selected patients effectively, to prevent morbid VTE in pregnancy [10].
Acquired Thrombophilias
Among the acquired thrombophilic disorders, anticardiolipin antibody, annexin V antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein1 (β2GPI) antibodies were more frequently seen in the Indian study on DVT in antenatal period [121]. The main acquired form of thrombophilia leading to increased risk of DVT in pregnancy is the antiphospholipid antibody syndrome (APLA). The patients with this disease present with venous and/or arterial thrombosis together with laboratory evidence for antibodies in blood that recognize anionic phospholipid–protein complexes [54]. The hypothesis of etiology of thrombosis in antiphospholipid syndrome is described elsewhere in this book.
In patients with APLA syndrome, vascular occlusions [23] are seen. Renal, celiac, and intracerebral artery stenoses [97, 98, 127] have been reported. In APLA syndrome, vascular changes reported include thrombosis, vascular intimal and smooth muscle hyperplasia, activation of platelets, and APLA antibodies-stimulating platelet aggregation [35]. Tissue factor activity by leukocytes is promoted by these antibodies. Protein C pathway is interfered by oxidation, thereby enhancing the anticoagulant activity of activated protein C [38]. Many patients with APLA syndrome are seen to have concurrent protein S deficiency as well [30]. The prevalence of APLA syndrome is estimated to be 5 % of the general population [21]. They are seen in >50 % of pregnancy-associated thrombosis.
Management of Deep Vein Thrombosis/Venous Thromboembolism
Diagnosis of DVT
Diagnosis of deep vein thrombosis (DVT) requires both clinical assessment and objective testing. The clinical features are mostly nonspecific. Investigations can either be falsely positive or negative.
Table 20.1 gives management principles of DVT/PE in general.
Table 20.1
Guideline for management of VTE
Assess risk of VTE for all pregnant women at booking or at the earliest opportunity |
Consider whether antenatal thromboprophylaxis is required |
Consider postnatal thromboprophylaxis liberally |
Reassess risk throughout the pregnancy and puerperium |
Make an individualized plan with the patient |
Ensure all women mobilize early postpartum and the puerperium |
Avoid dehydration all through pregnancy; encourage GCS usage liberally |
The initial step in the diagnostic process is to stratify patients into risk assessment categories using a validated clinical model:
High risk
Intermediate risk
Low risk
Refer Table 20.2 for risk categorization of VTE in pregnancy.
Table 20.2
Risk Assessment of VTE in Pregnancy
High risk: |
Prior VTE is the most important risk factor, especially when unprovoked/estrogen related |
Previous recurrent VTE >1 |
The inherited and acquired thrombophilias. Family history of thrombophilia is identified in less than 50 % of patients with unprovoked VTE [10] |
Intermediate risk: |
Single previous VTE with no family history of thrombophilia |
Thrombophilia + no previous VTE |
Low risk: |
Elective Cesarean delivery, which has twice the risk as vaginal delivery [108] |
Obesity, BMI >30 |
Age >35 years |
Smoking |
Current systemic infection |
Parity >3 |
Long-distance travel |
Dehydration, prolonged immobilization in pregnancy |
Multiple pregnancies |
Premature delivery |
ART |
Preeclampsia |
Screening for thrombophilias should be done if the results are likely to alter management of DVT. Screening is unnecessary when clinical suspicion of DVT is very high.
For all risk categories:
Consider clinical surveillance, encourage mobilization, and avoid dehydration.
If with intermediate or high risk, consider the following:
Graduated compression stockings (GCS), intermittent pneumatic compression (IPC) if hospitalized, and low-molecular-weight heparin (LMWH) prophylaxis. Avoid dehydration at all stages of pregnancy.
Long-haul flights require particular counseling and probably DVT prophylaxis.
Management of VTE in pregnancy is by a tertiary team, where a referral system has to be developed. The tertiary management team comprises of multidisciplinary personals. This team includes obstetricians, senior physicians, hematologists, interventional radiologists, intensivists, and thoracic surgeons. Figure 20.2 is a flowchart of management of VTE. This is particularly relevant to Indian scenario.
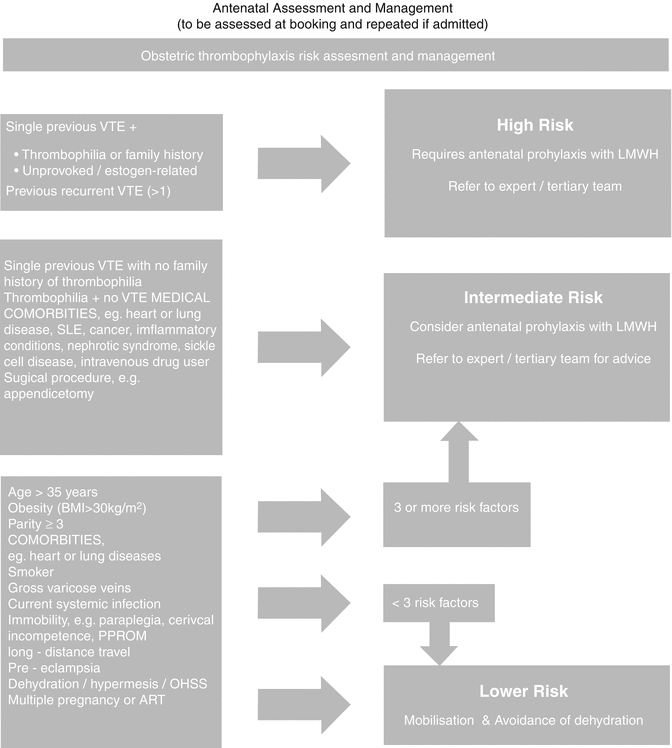

Fig. 20.2
Flowchart for the management of VTE in pregnancy (Adapted from “Thrombosis and Embolism during Pregnancy and the Puerperium, Reducing the Risk”, RCOG Green-top Guideline No. 37a. Nov. 2009) [113]
Signs and Symptoms of DVT and PE
Signs and symptoms of DVT and PE are generally nonspecific and hence overlooked. Refer to Table 20.3 for general signs and symptoms of VTE in pregnancy.
Table 20.3
Signs and symptoms of DVT and PE
Symptoms of DVT: |
Pain and swelling of the lower extremity |
Back pain and swelling of lower extremity in iliac vein thrombosis |
Symptoms of PE: |
Dyspnea – 82 % |
Abrupt onset of chest pain – 49 % |
Cough – 20 % |
The common presenting signs of PE: |
Tachypnea |
Crackles |
Tachycardia |
Patients with massive PE: |
Syncope |
Hypotension |
Pulseless cardiac electrical activity |
Death |
Principles of Treatment of DVT in Pregnancy
Pregnant women are more prone to have DVT. The clots form mostly in the lower limb veins and can break up to form emboli. These emboli can move to the lungs causing pulmonary embolism. This has serious consequences including maternal death. Anticoagulants are used to treat clots and are given to pregnant women with increased risk to clotting. These medications reduce the risk of further thrombosis and thereby reduce the risk of pulmonary embolism. An important complication of treatment with anticoagulants is hemorrhage. When a woman is anticoagulated, this risk of hemorrhage is present all through the pregnancy, i.e., in antepartum, intrapartum, and postpartum period.
During pregnancy heparin is the most common anticoagulant used, either the conventional unfractionated heparin (UFH) which is cheaper or the low-molecular-weight heparin (LMWH). Neither of these cross the placenta, and both have been shown to be safe during pregnancy. Oral anticoagulants like warfarin are generally considered unsafe in pregnancy as it may affect the fetus.
History and Physical Examination of DVT
Diagnosis of first episode of DVT is often difficult as most of the signs and symptoms are atypical and are seen commonly in pregnancy with non-thrombotic conditions as well. Symptoms like mild tachypnea, dyspnea, tachycardia, lower extremity edema, and cramps are common in most pregnant women. Hence, diagnosis of VTE by physical examination is frequently inaccurate or overlooked.
The two most common symptoms of DVT are unilateral pain and swelling of the lower extremity. Among the 80 % of pregnant women who experience these symptoms, only a few have true DVT. So also among 70 % of women with dyspnea, only a few have PE.
Examination findings of the following are important for the diagnosis of DVT:
Mid-calf circumference difference of ≥2 cm
Symptoms in the left lower extremity
First trimester presentation
Since the risks of VTE are increased in pregnancy and postpartum and the morbidity and mortality are great, a low threshold for initiation of evaluation with the above findings is recommended for the prevention of serious catastrophe. Until excluded, these women should begin anticoagulation therapy.
History and Signs and Symptoms of Pulmonary Thromboembolism
Risk assessment is essential, as deaths due to PE are mainly from overlook or not having clinical suspicion [11, 47] of the condition. This should start prepregnancy.
Clinical signs and symptoms of PE as with DVT are nonspecific. The classic symptoms of PE are dyspnea (82 %), abrupt-onset chest pain (49 %), and cough (20 %) and sometimes hemoptysis. The most common presenting signs are tachypnea, crackles, and tachycardia.
Pulmonary embolism is more often fatal, has a higher recurrence rate, and presents with less specific symptoms in comparison to DVT. Most of these signs and symptoms are common in “normal” pregnancies. PE is an enigma in pregnancy, in that all these signs and symptoms are rarely seen together. PE is usually a consequence of DVT. About 40 % of patients with proximal DVT are found to have an associated pulmonary embolism by lung scan; about 70 % of patients presenting with pulmonary embolism are found to have DVT in the legs [84].
Diagnosis is a real challenge to the clinician [126]. The clinician has to make an accurate judgment of this life-threatening condition. Therefore, if there is a suspicion of PE, anticoagulation therapy and appropriate immediate diagnostic testing should be performed until the diagnosis is made or ruled out as early as possible [69]. Patients with massive PE may present with syncope, hypotension, pulseless cardiac electrical activity, or death.
Electrocardiogram in PE
An electrocardiogram pattern suggestive of pulmonary embolism is a right ventricular strain and the S1Q3T3 [116]. These findings are mostly nonspecific and infrequent. Seventy percent of patients with PE have nonspecific ECG abnormalities.
Laboratory Evaluation for DVT/PE
D-dimer value of <500 ng/mL has been shown to have 99 % negative predictive value in patients with low and intermediate probability for VTE in the nonpregnant patients. D-dimer levels are increased in pregnancy if there is a concomitant problem such as preeclampsia [124]. At term and in the postnatal period, in most healthy pregnant women, D-dimer levels are raised [42]. The specificity of this test in pregnancy is hence low. In spite of that, it remains a test with good negative predictive value even in pregnancy [124]. DVT may be safely excluded if the D-dimer is negative [18] and the compression duplex ultrasonography (CUS) is normal. The sensitivity of compression duplex ultrasonography test is 100 % when put together with a low D-dimer test value.
Other laboratory testing, e.g., cardiac enzyme, arterial blood gas analysis, etc. [112], are useful to rule out possible differential diagnosis to PE. Before anticoagulation therapy is initiated, a full blood count, coagulation profile, urea, creatinine, electrolytes and liver function tests [113] should be performed. Performing thrombophilia screening for an acute episode of PE prior to treatment is not recommended.
Imaging in PE
A chest X-ray is the first image to be taken in suspected PE. CXR helps for differential diagnosis of other pulmonary conditions like pneumonia, pneumothorax, or lobar collapse. The most common chest radiography findings associated with pulmonary embolism are enlarged pulmonary arteries, peripheral wedge of airspace opacity which implies lung infarction (atelectasis or parenchymal density), pleural effusion, regional oligemia, and elevation of a hemidiaphragm. In several cases, CXR maybe normal. They are again nonspecific [39]. If CXR is normal, a bilateral CUS of lower limbs should be performed. If both tests are negative, but clinical suspicion is high, a ventilation–perfusion (V/Q) lung scan has to be performed. This can also detect other pathologies like a dissecting aorta. A computed tomography pulmonary angiography (CTPA) is the diagnostic test of choice when the technology is available and appropriate for the patient [85]. Even when the tests come negative, anticoagulation treatment should be continued if the clinical suspicion is high [5]. Magnetic resonance direct thrombus imaging (MRDTI) may be performed if the diagnosis still remains uncertain [107].
It is necessary to understand that the diagnostic strategy for pulmonary embolism (PE) during pregnancy is not based on strong evidence. Neither is it accepted unanimously. Most of the clinical scores are not validated. The diagnostic value of D-dimer is low and is rarely negative in pregnant women.
Imaging for DVT
The initial test of choice in the evaluation of DVT is compression duplex ultrasound (CUS) of the lower extremity veins [8, 74, 79, 86, 90, 100]. Sensitivity and specificity for proximal lower extremity DVT is more than 95 % for CUS. CUS is to be performed with the patient in the left lateral decubitus position. Doppler analysis of flow variation during respiration needs to be assessed as well, so as to maximize the study’s ability to diagnose pelvic DVT [5]. Veins have to be easily compressible and collapse completely. The normal venous blood flow should be spontaneous and phasic; cease with the Valsalva maneuver, and show augmentation with distal compression. Absence of this usually indicates the presence of a substantial clot (Fig. 20.3).
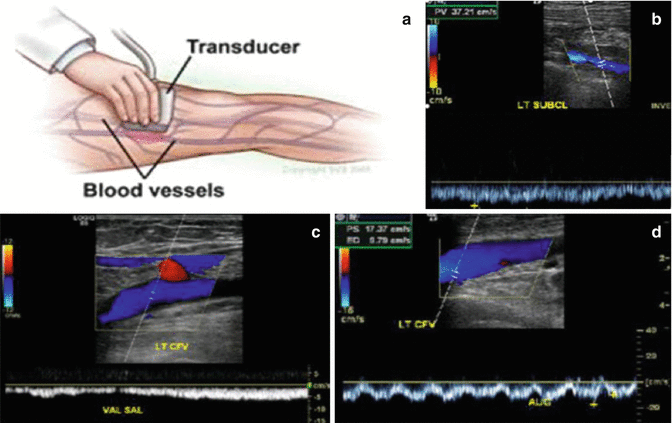

Fig. 20.3
Compression duplex ultrasound (CUS). (a) Patient in the left lateral decubitus position, flow variation during respiration needs to be assessed. http://www.vascularweb.org/vascularhealth. (b) Veins have to be easily compressible and collapse completely. Blood flow should be spontaneous and phasic, cease with Valsalva maneuver, and show augmentation with distal compression. (c) Duplex ultrasound showing abnormal Valsalva response. (d) Duplex ultrasound showing absence of augmentation with distal compression. (b–d) http://www.surgery.wisc.edu/referring-physicians
If CUS results are negative and if we have no suspicion of iliac (pelvic) vein thrombosis (usual symptoms are back pain and swelling of lower extremity), she may be left for routine observation. CUS can be done with reasonable accuracy for pelvic vein thrombosis in the first and second trimesters of pregnancy and with difficulty in the third trimester.
If the study is equivocal or abnormal, or if pelvic vein thrombosis is suspected, further evaluation is recommended. Magnetic resonance venography is the image of choice [70, 90, 107]. Conventional contrast venography may also be performed if MRI is not available [86]. The risk of radiation exposure to the fetus has to be discussed with the patient in such instances [85]. The choice of imaging testing is based on availability and in consultation with the radiologist (Table 20.4).
Table 20.4
Diagnosis of lower extremity deep vein thrombosis
Diagnosis of lower extremity deep vein thrombosis |
|---|
Compression ultrasound (US) is highly sensitive and specific for the detection of deep vein thrombosis (DVT) in the upper leg |
Lower extremity US can give indirect evidence of pelvic DVT. However, MR venography is recommended for direct diagnosis of suspected pelvic DVT |
US is not sufficiently sensitive to rule out thrombosis below the knee, and, if clinical suspicion remains high, US examination should be repeated after a week because of the danger of thrombus propagation into the thigh veins |
CT pulmonary angiography combined with CT venography of the lower extremity is recommended for patients with symptoms of pulmonary embolism to detect emboli in the lung and to screen for DVT |
Treatment of DVT in Pregnancy and the Puerperium
If clinical suspicion of DVT or PE is high, empirical treatment with LMWH should be given until the diagnosis is excluded by objective testing. LMWH is considered equally effective as unfractionated heparin in the initial treatment of VTE. Advantages of LMWH over UFH include the following: it does not cross placenta just as UFH, it lowers the risk of hemorrhagic complications, it lowers mortality compared to UFH, and there is no risk of heparin-induced thrombocytopenia [9, 36, 40, 66, 72, 93, 99]. Different LMWH preparations have been compared for their efficacy in the treatment of VTE in pregnancy, and the data are now available [55, 71, 96, 106]. There seems to be no particular advantage of one preparation over the other. The risk of recurrent VTE after treatment with LMWH in pregnancy is comparable to that in nonpregnant state when VTE was treated with similar LMWH (1.15 % vs. 5–8 %) [52]. It is also comparable to patients treated with unfractionated heparin or coumarin, especially when followed up over 3–6 months of initial episode [128]. LMWH does not increase peripartum bleeding and hence particularly useful where hemorrhage accounts for major peripartum morbidity and even mortality. No case of heparin-induced thrombocytopenia has been recorded with LMWH [16, 128]. Heparin-induced osteoporosis [49] is hardly seen with LMWH.
To summarize, comparing LMWH to unfractionated heparin (UFH), LMWH decreased the risk of mortality, recurrent VTE, and hemorrhage. Disadvantages of LMWH include cost and longer half-life (Fig. 20.4).
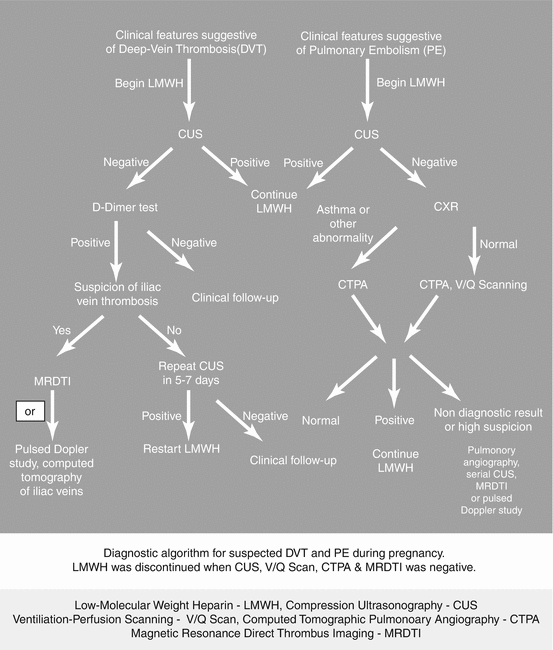

Fig. 20.4
Flow chart for the management of DVT/PE. LMWH low-molecular-weight heparin, CUS compression ultrasonography, V/Q Scan ventilation–perfusion scanning, CTPA computed tomographic pulmonary angiography, MRDTI magnetic resonance direct thrombus imaging (Courtesy, Medscape CME and Education, Thromboembolism in Pregnancy, April 2014) [34]
The Therapeutic Dose of LMWH in Pregnancy
Subcutaneous low-molecular-weight heparin (LMWH) is the preferred treatment [118] for most patients with acute VTE [10]. LMWH should be given daily in two subcutaneous divided doses [80]. The most commonly used LMWH is enoxaparin. Enoxaparin 1 mg/kg twice daily or dalteparin 100 units/kg twice daily is the recommended dose. Tinzaparin 175 units/kg is also considered equivalent in the treatment of VTE in pregnancy [49]. This is called weight-adjusted, fixed-dosage regime. Routine platelet count monitoring is not required in women who receive only LMWH. Occasionally, the dosing of LMWH may have to be monitored and adjusted by anti-Xa assay because of the effects of increased plasma volume and glomerular filtration rate in pregnancy [19]. Monitoring anti-Xa is expensive. But it is beneficial in extremes of body weight [61], (below 50 kg or above 90 kg) or for patients with renal disorders or if there is a recurrent VTE episodes. Deciding the dose of LMWH with anti-Xa measurement is called adjusted-dose regime. If anti-Xa level is measured, it should be 3–6 h after the third or fourth dose of enoxaparin or third or fourth dose after dose adjustments. The target is to achieve an optimal peak anti-Xa level of 0.5–1.2 IU/ml. The LMWH dose may have to be increased or decreased 10–25 % to achieve the optimal anti-Xa level [10, 80].
For patients greater than 150 kg, UFH maybe preferred, or otherwise, a closer monitoring of anti-Xa levels should be performed to ensure therapeutic effect. Unfractionated heparin (UFH) may also be preferred if the patient is likely to have immediate surgery or delivery because of its shorter half-life and its reversibility with protamine. However, due to cost, some patients may have limited access to LMWH especially in a country like India. UFH should not be denied to such patients. Monitoring of heparin therapy is usually by measurement of the activated partial thromboplastin time (aPTT) [10]. A therapeutic range of aPTT ratio (international normalized ratio or INR) of 1.5–2.5 is recommended [50]. UFH is administered by IV bolus, followed by IV infusion, with titration of the dose to a standard aPTT. The heparin infusion is typically increased or decreased by 10–30 % to titrate to goal aPTT [10].
After reaching a therapeutic and stable aPTT, the heparin can be converted to either subcutaneous UFH or LMWH. The disadvantage of subcutaneous UFH is that it is less predictable for anticoagulation as dosing variability exists to maintain therapeutic response. The platelet count needs to be monitored every 2–3 days from day 4 to day 14 or until heparin is stopped, whichever occurs first [10, 34, 112].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


