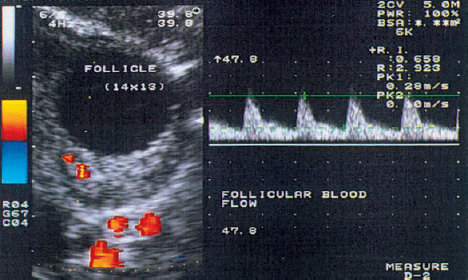
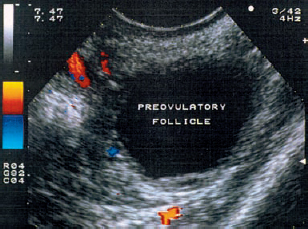
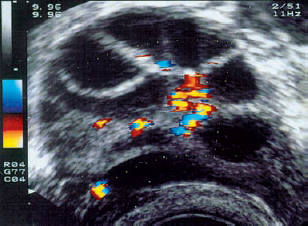
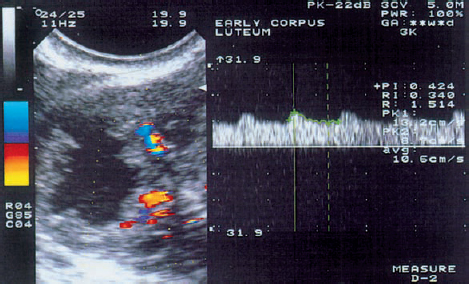
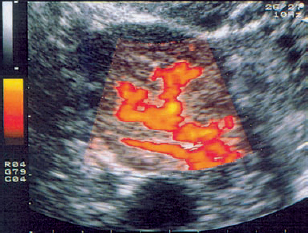
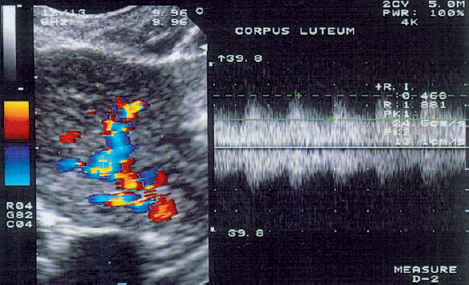
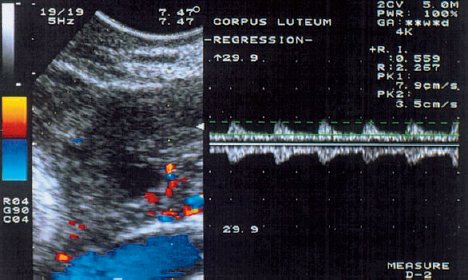
10 Doppler Ultrasound Examinations from Folliculogenesis to Early Pregnancy The introduction of transvaginal color Doppler sonography has dramatically enhanced our understanding of early human development. This study permits the noninvasive assessment of blood flow in the corpus luteum and the changes in uterine blood flow that occur in association with pregnancy. For example, when corpus luteum blood flow was analyzed in normal and abnormal pregnancies, no difference was found between ectopic pregnancy and normal early pregnancy, but the resistance and pulsatility indices in cases of threatened abortion, incomplete abortion, and missed abortion were found to be significantly higher than in normal pregnancies. In several studies, color Doppler imaging has shown a progressive decline in the resistance and pulsatility indices from the main branch of the uterine artery to the spiral arteries with advancing gestational age. All the vessels examined showed a decrease in flow resistance as the pregnancy progressed. Transvaginal color Doppler ultrasound has provided a unique opportunity to study both the morphology and physiology of human life from conception to implantation. The follicular cells become luteinized after ovulation. Many biochemical, morphological, and circulatory changes take place during this transformation process, and a large proportion of these changes can be investigated by transvaginal color Doppler scanning. After fertilization of the oocyte, the embryo is transported into the uterine cavity, where it implants under favorable local and hormonal conditions and develops into a new individual. Transvaginal ultrasound with color Doppler imaging and blood flow analysis during these phases permits a detailed examination of the small vessels that supply the preovulatory follicle, corpus luteum, and endometrium. The ovary and ovarian blood flow. Transvaginal ultrasound scanning also permits an increasingly precise visualization of ovarian morphology8. The addition of color Doppler ultrasound makes it easier to measure the sequential changes in the arteries that supply the ovaries during the course of the cycle2,3,4,24,38. The highest flow resistance is measured on the first day of the cycle, the lowest on the day of the LH (luteinizing hormone) peak3,4. Follicular blood flow. The surveillance of folliculogenesis with ultrasound has become a well-established modality in routine clinical practice. The blood flow at the periphery of the follicle can be clearly demonstrated with transvaginal color Doppler ultrasound3,4. Normally, a follicular flow velocity waveform can be recorded when the dominant follicle has reached a diameter of 10 mm. The resistance index (RI) is approximately 0.54 in the periovulatory period24 (Fig. 10.1). The RI begins to decline two days before ovulation and reaches its lowest value when ovulation occurs (0.44 ± 0.04). Measurements taken shortly before ovulation typically show a sharp rise in the peak systolic blood flow velocity with a relatively constant resistance index. This observation may result from dilatation of the newly formed vessels between the vascularized theca cell layer and the hypoxic granulosa cell layer of the follicle12. Normal ovulation depends upon changes in the oxygen partial pressure and the effect of these changes on cellular function (Fig. 10.2). Transvaginal sonography with color Doppler imaging is becoming the method of choice for investigating the subtle blood flow changes that take place in the normally and abnormally functioning ovary (Fig. 10.3). Fig. 10.1 Transvaginal scan of the dominant follicle three days before ovulation indicates a moderate vascular resistance (RI = 0.65). Color Doppler ultrasound can define the small vessels that supply the follicle. Fig. 10.2 Triangular outline of the mature follicle. Fig. 10.3 Image of an ovary with numerous follicles after prior ovulation induction with hCG. Each follicle is ringed by vascular structures that can be visualized with Doppler ultrasound. Developmental stages. The cells of the follicular wall undergo structural and functional changes as they develop into the components of the corpus luteum17. Microscopically, the corpus luteum undergoes four developmental stages: proliferation (Fig. 10.4), vascularization, maturation, and regression. In the proliferative phase, the theca interna becomes infolded while its vascular channels become greatly dilated. Vessels sprout from the endothelium, penetrate the granulosa cell layer, and enter the hemorrhagic cavity of the ruptured follicle. The vascularization phase is characterized by a rapid organization of the blood-filled cavity of the ruptured follicle (Figs. 10.5, 10.6). With increasing maturation, the theca cells and the lutein cells derived from the granulosa acquire vacuoles and are physiologically active. The mature corpus luteum measures 1–3 cm in section and displays low resistance signals (average RI = 0.43). Regressive changes in the corpus luteum can be seen as early as day 23 of the cycle (Fig. 10.7). Typically these changes are marked by a decreased blood flow velocity and a rising resistance index (average RI = 0.49). If pregnancy occurs, the corpus luteum persists in response to hCG (human chorionic gonadotropin) synthesized by the trophoblasts and produces progesterone to support the developing embryo. The corpus luteum begins to regress after 10 weeks’ gestation, at which time the placenta assumes the task of progesterone production. Regression of the corpus luteum is complete by the 16th week of gestation29. Fig. 10.4 Early development of the corpus luteum in a transvaginal color Doppler scan. Note the dilated vessels at the periphery of the collapsed follicle (left). A decreased vascular resistance (RI = 0.40) is demonstrated (right). Fig. 10.5 Transvaginal power Doppler image showing increased vascularization of the corpus luteum. Fig. 10.6 Typical blood flow pattern of the mature corpus luteum: high blood flow velocity and low vascular resistance (RI = 0.47). Fig. 10.7 Transvaginal scan of the ovary in the late luteal phase (left). During the luteal regression phase, diastolic blood flow is decreased and the RI is increased (right). The use of Doppler ultrasound during these stages can supply interesting information15,36. The noninvasive Doppler assessment of blood flow velocity in the corpus luteum provides direct information on the arterial perfusion of the ovary26,32. Transvaginal color Doppler can demonstrate even small vessels in the ovary. The results are very precise and reproducible. Uterine blood supply. Implantation and subsequent pregnancy in humans are dependent upon complex changes in the uterine artery and its branches in the myometrium and endometrium. The blood flow changes in the uterine vessels during a reproductive cycle are important for implantation and for the development of a uteroplacental blood supply. Transvaginal color Doppler gives a detailed view of the genital organs and of most blood flow changes that occur in these organs19. The color-flow signal from the uterine artery can be identified lateral to the cervix (Fig. 10.8). After passing through the outer third of the myometrium, the uterine arteries divide into the vascular plexus of the arcuate arteries, which are distributed within the uterus18. The radial arteries arise from this plexus and run toward the uterine cavity (Fig. 10.9). On reaching the junction of the myometrium and endometrium, they are called the spiral arteries. Transvaginal color Doppler is an efficient tool for analyzing the waveforms of the radial and spiral arteries. It is well known that the endometrium undergoes profound structural and functional changes during the course of the cycle9,11,18,33. Demonstrable histological changes include the vigorous development of essential blood vessels, especially the spiral arteries, during the menstrual cycle (Fig. 10.10
Overview
Monitoring Folliculogenesis
Development of the Corpus luteum
Changes in Endometrial Blood Flow
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree