Normal liver physiology in pregnancy
Outside pregnancy, the liver receives up to 25–35% of the cardiac output [1] and this surprisingly does not change significantly during pregnancy. The size of the liver does not increase during pregnancy and it is indeed more difficult to palpate as pregnancy progresses due to its posterosuperior displacement by the enlarging uterus. A palpably enlarged liver is therefore abnormal in pregnancy and may indicate congestion, infiltration or malignancy.
Metabolic, synthetic, and excretory functions of the liver are affected by the increased levels of estrogen and progesterone in pregnancy [2]. The serum albumin concentration decreases during pregnancy and reaches a nadir towards term secondary to the increased plasma volume. Abnormal liver function tests occur in 3–5% of pregnancies with many potential causes. Table 9.1 summarizes the changes in liver function occurring during pregnancy.
Table 9.1 Liver function tests in pregnancy
| Test | Result |
| Bilirubin | Unchanged or slightly decreased |
| Aspartate transaminase (AST) | Unchanged initially but 25% decrease by third trimester |
| Alanine transaminase (ALT) | Unchanged initially but 25% decrease by third trimester |
| Gamma-glutamate transaminase (GGT) | Unchanged or slightly decreased |
| Alkaline phosphatase | 2–4-fold increase in the third trimester |
| Cholesterol | Twofold increase |
| Triglycerides | 2–3-fold increase |
| Globulin | Increase in alpha and beta globulins |
The most significant changes are the decreased liver transaminase (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) levels to 25% pre-pregnancy levels by the third trimester. Alkaline phosphatase levels can increase up to four times [3] and do not indicate an obstructive problem within the hepatobiliary tree. The rise is caused by leakage of placental alkaline phosphatase into the maternal circulation and increased maternal bone turnover. Alkaline phosphatase levels may remain elevated for up to 6 weeks post partum. Plasma cholesterol levels increase by 50% by the third trimester with triglyceride levels increasing 2–3-fold from nonpregnant values [4].
Normal pregnancy in a healthy woman is frequently associated with the appearance of both spider nevi (vascular skin lesions with a central arteriole feeding outwardly radiating thin-walled blood vessels) and palmar erythema. These findings are also seen in patients with chronic liver disease but are not of clinical significance in the context of pregnancy.
Maternal hyperbilirubinemia and the fetus
Excretion of fetal bilirubin occurs through transplacental transfer into the maternal circulation although the volume of fetal bilirubin is small enough that maternal serum levels of bilirubin are not significantly changed from the normal range for nonpregnant patients. Fetal bilirubin is then conjugated in the maternal liver before being excreted into bile. Elevated levels of unconjugated bilirubin and its metabolites seen in maternal liver failure do not appear to have a deleterious effect on the long-term neurodevelopmental status of the offspring [5,6]. This is in marked contrast to neonatal hyperbilirubinemia in hemolytic disease of the newborn which can result in bilirubin deposition in the brainstem, causing kernicterus [7].
Hepatitis A virus
Hepatitis A virus (HAV) infection usually results in a self-limited icteric illness in adults and an asymptomatic infection in children but, unlike hepatitis B or C, does not cause chronic infections. It is not an important cause of maternal or neonatal morbidity, yet it can be transmitted from mother to child. HAV vaccine is highly effective and vaccination during pregnancy is appropriate for women who may be at high risk for exposure (e.g. ongoing exposure to clotting factor concentrates, occupational exposure to HAV or unavoidable travel to countries with high endemicity of hepatitis).
Virology
Hepatitis A virus is a small nonenveloped RNA virus, similar to other enteroviruses; the lack of lipid envelope makes it relatively heat and acid resistant, so it can remain infectious in the environment for weeks. Humans are the only important reservoir and there is only one known serotype.
Epidemiology
Hepatitis A virus is found worldwide but the prevalence is directly related to sanitary conditions. Infections are acquired early in life in highly endemic areas of the world (parts of Africa, Asia, Central and South America) whereas in low endemic areas (Canada, USA, Western Europe and other developed countries), most adults remain susceptible. Targeted use of HAV vaccine during childhood in some developed countries has dramatically decreased the number of infections in all ages, as children likely were an important reservoir for transmission in the community [8]. Risk factors for infection include attendance at childcare centers, travel to high or intermediate prevalence areas, men who have sex with men and use of illicit drugs [9]. The incidence of acute disease in pregnancy was less than 1:1000 prior to introduction of HAV vaccine in the mid 1990s, but updated estimates are lacking.
Pathogenesis
Person-to-person transmission by the fecal–oral route is the primary means of HAV acquisition [10], usually through ingestion of contaminated foods and person-to-person contact. Once ingested, HAV replicates in the small bowel and the liver, is then excreted to the bile, and then shed in the stool in high quantities. There is a short-lived period of viremia and peak infectivity occurs during the 2 weeks before the onset of symptoms. At this time there is jaundice and liver enzyme elevation. HAV is not directly cytopathic to the liver.
Pathogenesis of mother-to-child transmission
Mother-to-child transmission of HAV is very rare. Only two cases of intrauterine transmission following maternal infection in the first trimester have been reported and resulted in fetal meconium peritonitis [11,12]. Maternal infection in the third trimester of pregnancy may result in asymptomatic neonatal infection and/or self-limited neonatal cholestasis [13–15]. Breastfeeding is not implicated in transmission [15].
Clinical presentation
Acute HAV infection in pregnancy has the same clinical presentation and prognosis as that in the nonpregnant adult; pregnant women do not appear to be more susceptible to this disease. The average incubation period lasts 2–7 weeks (mean of 4); it is followed by a mild illness consisting of fever, chills, anorexia, nausea and vomiting and then by the onset of dark urine, pale stools, jaundice and hepatomegaly. Transaminases are classically elevated by 10–100 times the normal range. Most symptoms and signs resolve within 3 weeks.
Complications include cholestasis in about 7% of patients [16] (i.e. prolonged jaundice, pruritus and fever), prolonged and relapsing disease present in 10–15% of cases, fulminant hepatitis in less than 1% of cases, and death. Mortality rate is low at 0.4% [17]. Chronic hepatitis A infection, as can be seen with hepatitis B and C, does not occur.
Other obstetric considerations
Hepatitis A virus is not a recognized teratogenic agent. In a recent retrospective report, acute HAV in the second half of pregnancy was associated with premature contractions and premature rupture of membranes [18].
Diagnosis
Diagnosis requires laboratory confirmation, as clinical presentation can be indistinguishable from other acute viral hepatitis. Anti-HAV IgM is the test of choice to diagnose acute infection given that it remains positive for 3–6 months only. Total anti-HAV antibodies are not clinically useful to diagnose acute infection because anti-HAV IgG persists for life.
Treatment and prevention
Treatment is supportive (see general treatment in HBV section).
Currently licensed HAV vaccines are inactivated killed virus vaccines. They are very effective at preventing infection and their risk to the developing fetus is expected to be very low. Therefore, vaccination during pregnancy is appropriate for women who may be at high risk for exposure, including chronic liver disease, ongoing exposure to clotting factor concentrates, occupational exposure to HAV or unavoidable travel to countries with high endemicity of hepatitis A. Patients visiting endemic areas should receive one dose of HAV vaccine at least 2 weeks before traveling, and a booster dose 6–12 months after the initial vaccination [19]. Equally important for any person traveling in areas with endemic hepatitis A is avoidance of uncooked fresh vegetables and shellfish, and access to clean water.
Prevention of maternal illness after a known exposure to HAV
Passive immunization with immunoglobulin within 2 weeks of exposure to HAV is effective in preventing 80–90% of HAV cases, and immunoglobulin use is safe in pregnancy [19].
Prevention of neonatal infection
Immunoglobulin at a dose of 0.02 mg/kg is recommended to prevent neonatal infection if maternal illness develops 2 weeks before or 1 week following delivery.
Infection control
Hospitalized patients with HAV do not require isolation or contact precautions unless incontinent. Frequent hand washing should be enough to prevent spread in the hospital setting.
Hepatitis B virus
Hepatitis B virus (HBV) accounts for close to 350 million chronic infections worldwide [20] and for an estimated 1 million deaths each year due to cirrhosis and hepatocellular carcinoma. Thirty to fifty percent of HBV chronic infections are acquired through the mother-to-child route, as acquisition in the neonatal period poses the highest risk for developing chronic HBV infection. Fortunately, HBV vaccination programs have dramatically reduced the occurrence of chronic HBV infection and its complications; in addition, most infections transmitted from mother to child can be prevented by timely recognition of maternal infection and neonatal active and passive immunization.
Virology
Hepatitis B virus is a small, double-stranded DNA hepatotropic virus, meaning that this virus primarily infects liver cells but does not directly kill them. Any cellular damage associated with the virus appears to be immune related. Its lipid envelope has a unique antigenic protein called hepatitis B surface antigen (HBsAg), which is overproduced and hence found free in the blood of infected patients. The inner core contains the genome, a polymerase, an inner nucleocapsid “core” antigen (HBcAg), essential for virus packaging, and the e antigen (HBeAg), which is related to infectivity. HBV cannot be cultured, so diagnosis relies on rapid virus detection (i.e. viral load) or serology. There are eight recognized serotypes, with distinctive rates of progression to chronic infection and cirrhosis, and different prevalence by geographic location.
Epidemiology
Hepatitis B virus infection is found worldwide, but the prevalence of chronic infection ranges widely from 0.1–2% in so-called low-prevalence areas like North America, Western Europe, Australia, and New Zealand to 10–20% in high-prevalence areas like South East Asia, China, and sub-Saharan Africa. Japan, Central Asia, the Middle East, Central America, and South America are intermediate-prevalence areas. Mode of transmission clearly differs among these geographic areas; mother-to-child transmission predominates in high-prevalence areas, whereas horizontal transmission, particularly in early childhood, accounts for most cases of chronic HBV infection in intermediate-prevalence areas. Unprotected sexual intercourse and intravenous drug use in adults are the major routes of spread in low-prevalence areas.
In the United States, acute hepatitis B occurs in approximately 1 in 1000 pregnancies. Prevalence of HBsAg among pregnant women in an urban setting has been reported as 5.79% for Asian Americans, 0.97% for African Americans, 0.6% of whites and 0.14% for Hispanics [21].
Transmission
Hepatitis B virus can be transmitted transplacentally, perinatally, by sexual contact, by parenteral inoculation (IV drug use or exposure to blood products) and by close person-to-person contact presumably by open cuts and sores, especially among children in highly endemic areas. Blood contains the highest concentrations of virus, but other body fluids (semen, saliva, cervical secretions) and leukocytes also contain high viral titers. Although breast milk from chronically HBV-infected mothers is known to contain the virus [22,23], breastfeeding does not seem to increase the risk of transmission to the neonate above that already posed by pregnancy and delivery [24]. In addition, appropriate neonatal immunoprophylaxis has been shown to eliminate any theoretical risk of transmission through this route [25].
General course outside pregnancy
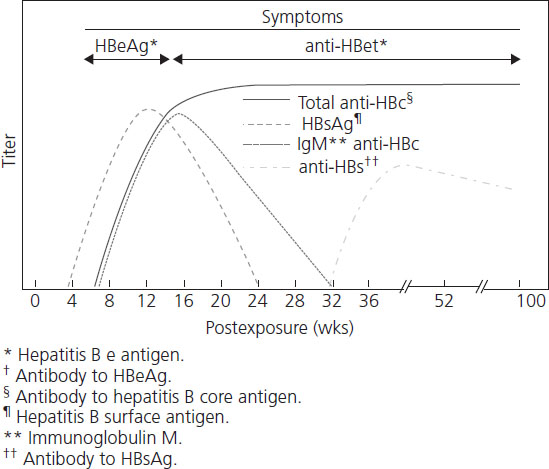
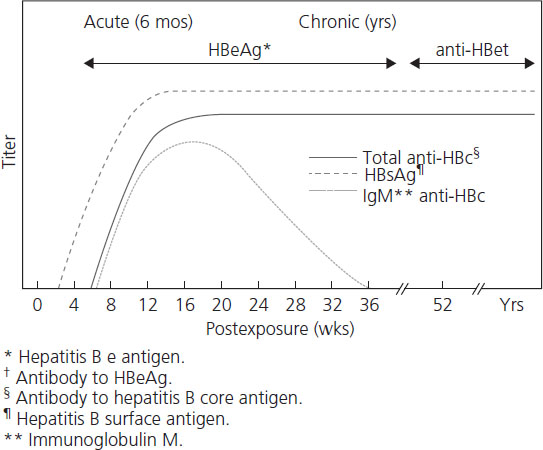
The natural history of hepatitis B is variable and still not completely understood. After initial infection, there is a 30–180-day incubation period followed by 30–90 days of acute hepatits. Only one-third of adults experience symptoms of acute hepatitis, while the majority (65%) have subclinical disease. Symptomatic acute hepatitis B syndrome presents no differently from other acute viral and nonviral hepatic diseases (including HELLP syndrome or acute fatty liver of pregnancy) and consists of malaise, fatigue, anorexia, nausea, vomiting, right upper quadrant discomfort, jaundice and tender hepatomegaly. The risk of developing symptomatic icteric hepatitis is inversely proportional to age, ranging from 0% among neonates to 80% of adults [26]. The diagnosis is usually made serologically with the demonstration of HBsAg, HBeAg or HBV DNA in maternal blood. Liver enzymes (AST and ALT) are elevated in both symptomatic and asymptomatic patients, although transaminase levels are rarely greater than 2000 IU/L. ALT is characteristically higher than AST. Figures 9.1 and Figures 9.2 and Table 9.2 review the typical progression of serologic testing in acute and chronic hepatitis B.
Table 9.2 Interpretation of hepatitis B serology
Adapted from: A comprehensive immunization strategy to eliminate hepatitis B virus transmission in the US: recommendations from the Advisory Committee on Immunization Practices. Part 1. Immunization of infants, children and adolescents. MMWR 2005;54:RR-16.
| Tests | Results | Interpretation |
| HBsAg | Negative | Susceptible |
| Anti-HBc | Negative | |
| Anti-HBs | Negative | |
| HBsAg | Anti-HBc | Immune due to natural infection |
| Anti-HBc | Positive | |
| Anti-HBs | Positive | |
| HBsAg | Negative | Immune due to hepatitis B vaccination |
| Anti-HBc | Negative | |
| Anti-HBs | Positive | |
| HBsAg | Positive | Acutely infected |
| Anti-HBc | Positive | |
| IgM anti-HBc | Positive | |
| Anti-HBs | Negative | |
| HBsAg | Chronically infected | Positive |
| Anti-HBc | Positive | |
| IgM anti-HBc | Negative | |
| Anti-HBs | Negative | |
| HBsAg | Negative | Interpretation unclear: four possibilities: |
| Anti-HBc | Positive | 1. resolved infection (most common) |
| Anti-HBs | Negative | 2. false-positive anti-HBc and therefore susceptible |
| 3. “low level” chronic infection | ||
| 4. resolving acute infection |
• HBsAg is the hepatitic B surface antigen and its presence indicates that the person is infectious.
• Anti-HBc is the IgG antibody to hepatitis B core antigen and appears early on with acute hepatitis B infection and stays for life. Its presence indicates infection with hepatitis B without a specific time frame.
• IgM anti-HBc is the IgM antibody to hepatitis B core antigen and is presence indicates acute infection with hepatitis B within the past 6 months.
• Anti-HBs is the IgG antibody to hepatitis B surface antigen and is presence indicates recovery from (or vaccination for) hepatitis B infection.
Over 90% of immunocompetent adult patients clear the infection and experience complete resolution after acute illness. Between 0.1% and 0.5% of patients with acute hepatitis B will develop a poorly understood acute fulminant hepatitis believed to be due to massive immune-mediated cell lysis of infected hepatocytes [27]. Another 5% of all infected adults (2% of women and 7% of men) will not “clear” the virus and develop chronic hepatitis B. Chronic infection is defined as persistence of HBsAg for more than 6 months or HBsAg without anti-Hbc IgM. Rates of chronicity are higher in newborns (90%) and children (30%) and in immune-deficient individuals. Some of the variation in outcome of HBV infection may relate to the genetic heterogeneity of the virus. Eight genotypes of HBV have been described, genotype A being most common in the United States and Northern Europe, B and C in Asia, and D in Mediterranean countries and the Middle East. Chronic infection with HBV genotype C appears to have a poorer prognosis and greater likelihood of cirrhosis and hepatocellular carcinoma (HCC) than genotype B [28–30].
The transition from acute to chronic infection represents a failure of immune clearance of virus-infected cells and is characterized by the persistence of high levels of HBV DNA and HBeAg in serum. The course of chronic hepatitis B is highly variable. Typical chronic hepatitis B is marked by the presence of HBeAg and high levels of HBV DNA with variable elevations in ALT and histologic activity. Patients are generally asymptomatic but may experience mild “flu-like” symptoms, arthralgia, malaise, anorexia, vomiting or occasionally a skin rash. The main long-term risks of chronic hepatitis B include hepatocellular carcinoma and cirrhosis with liver failure.
Approach to patients with possible chronic hepatitis B outside pregnancy
Patients who are HBsAg positive (without IgM antibodies to hepatitis B core antigen to suggest that the patient has an acute hepatitis B infection) should undergo an evaluation for chronic hepatitis B. This includes full liver function testing (AST, ALT, bilirubin, alkaline phosphatase, albumin, international normalized ratio (INR)) and testing for HBeAg, HBeAb and hepatitis B viral load (HBV DNA by polymerase chain reaction (PCR)). Patients should also be tested for hepatitis C and HIV, both of which are transmitted in a similar manner to hepatitis B and have implications for treatment of chronic hepatitis B. It is important to be aware that normal liver function testing on one occasion does not preclude chronic hepatitis. These patients should be sent, with these results, to a clinician who routinely cares for patients with chronic hepatitis B. Decisions will then be made as to whether the patient warrants observation, liver biopsy and/or treatment based on a wide range of clinical criteria. In some patients, treatment decisions may need to be deferred while the patient is followed over time to determine if they have an inactive carrier state or if they will clear the virus on their own.
Two major types of antiviral treatment are currently used. These include interferon alpha (IFN-alphia or PEG-IFN-alpha) and nucleoside or nucleotide analogs such as lamivudine.
Outside pregnancy the major goal of treatment for hepatitis B is to prevent progression of the disease to cirrhosis, endstage liver disease or hepatocellular carcinoma. Suppression of HBV replication can reduce histologic chronic active hepatitis and therefore the subsequent risk of cirrhosis and hepatocellular carcinoma. Extrahepatic manifestations of hepatitis B such as glomerulonephritis or polyarteritis nodosa also require treatment. If the disease has not progressed to cirrhosis, then prevention of progression to advanced fibrosis or cirrhosis is desirable. If cirrhosis has developed then preventing decompensation or hepatocellular carcinoma or death is important. The endpoints of treatment have not been clearly defined and differ in HBeAg-positive versus -negative disease. Improvement can be defined if HBV replication is suppressed to <1×104 copies/mL (2000 IU/mL), with an accompanying improvement in serum ALT and hepatic inflammation. Newer agents are capable of suppressing most patients to fewer than 1×103 copies/mL (200 IU/mL) or even to levels undetectable by current PCR assays (i.e. <200 copies/mL (50 IU/mL)).
Hepatitis B in pregnancy
Screening and vaccination
All pregnant women should be screened for HBV infection at their first prenatal visit by determination of their HBsAg status [31,32]. If the initial HBSAg is negative but the patient has an ongoing high risk of infection (countries with high prevalence, intravenous drug use, multiple sexual partners, multiple transfusions, immunosuppressed patients, bisexual, hepatitis B-positive partner, healthcare workers, etc.), then patients should be screened again in late pregnancy. The aim of prenatal screening is to identify infected women whose babies can then be offered neonatal immunoprophylaxis. Women at high risk for acquiring hepatitis B infection who are HBsAg negative and have not been vaccinated for hepatitis B should be tested for anti-HBsAg. If they show no immunity to hepatitis B, advice should be given regarding risk factor modification and the patient should be offered hepatitis B vaccine, generally after the first trimester. The vaccine is not a live virus and has not been associated with adverse effects in pregnancy but because of limited published data about its use in pregnancy (even in the first trimester), its use in pregnancy is generally recommended only for pregnant women at ongoing risk for hepatitis B virus infection, including occupational risks (e.g. healthcare worker), lifestyle risks (e.g. history of intravenous drug use, sexually transmitted diseases, more than one sexual partner in the preceding 6 months), co-morbidities that place the patient at risk of acquiring hepatitis B (e.g. hemophilia and hemodialysis) and environmental risks (e.g. prison inmates, refugees, international travelers).
Approach to the HBsAg-positive patient in pregnancy
The most common problem that obstetricians will encounter in screening for hepatitis B is an asymptomatic pregnant woman found to be hepatitis B surface antigen positive at the time of her first prenatal visit. The question then arises as to whether this represents a chronic carrier state or an acute infection. If there is no history of recent exposure or any symptoms of acute hepatitis, most of these cases will represent a chronic carrier state. When there is uncertainty about whether the patient has acute or chronic hepatitis B, IgM antibodies against hepatitis B core antigen can be obtained. These antibodies should be elevated in acute hepatitis B but not in chronic hepatitis B. For patients with both acute and chronic hepatitis B in pregnancy, it is important to plan for neonatal prophylaxis. For patients with chronic hepatitis B infection, it is also important to have the mother fully assessed as to whether she herself will require treatment after delivery. This assessment is best carried out by a practitioner who routinely cares for patients with chronic hepatitis B.
Patients should be informed that there is no evidence that pregnancy alters the clinical course of either acute or chronic hepatitis B. Acute or chronic infection with HBV is also not a cause of congenital malformations [33], nor of pregnancy loss or stillbirth.
Evidence for the efficacy of antiviral therapy for chronic HBV during pregnancy is very limited and its use is generally deferred until after pregnancy. Lamivudine has been used to treat HBV in pregnancy among chronically infected [34] or highly viremic women [35] and is reported to decrease mother-to-child transmission and delayed active hepatitis in mothers, but there are no data from randomized trials to routinely recommend its use at this time. It is unlikely that delays in treatment for the duration of gestation will have significant adverse effects on long-term prognosis for most patients and proper postpartum prophylaxis for the newborn is highly effective at preventing mother-to-child transmission of hepatitis B.
Pathogenesis of mother-to-child transmission
Hepatitis B virus mother-to-child transmission happens by two different mechanisms: in utero infection (transplacental) and direct inoculation during delivery, also called perinatal.
Perinatal transmission of HBV is the most frequent route of transmission to the newborn, as the fetus is exposed to infected blood and other fluids present in the birth canal, and can result from both maternal chronic HBV infection or acute maternal HBV infection in the third trimester.
Maternal HBV replicative status determines the mother’s infectivity, i.e. her risk of transmission to the infant. In the absence of HBV active and passive neonatal immunization, the risk of transmission for infants born to a “highly infectious” mother, e.g. HBsAg positive plus HbeAg positive and HBV DNA positive, is 85–90%, whereas the risk for an infant born to a “less infectious” mother, e.g. HBsAg positive but HbeAg negative, is only 10–32% [36,37].
Transplacental HBV transmission is very unusual except in the setting of acute HBV infection during the third trimester, when infection of placental capillaries transmits HBV to the fetus. HBV placental infection propagates from cell to cell, starting in the decidual cells, proceeding then to the fetal villous capillary endothelial cells and eventually reaching the fetal circulation. It is not interrupted by neonatal hepatitis B vaccination and may account for the small percentage of infants who are not protected by hepatitis B immunoglobulin.
Children born to mothers who are HBsAg positive at delivery, either from chronic hepatitis B infection or from acute HBV in the last trimester of pregnancy, should receive active (i.e. HBV vaccine) and passive (i.e. hepatitis B immune globulin – HBIG) immunization as follows:
- HBV vaccine: first dose given within 12 hours of birth and the second and third doses at 1 and 6–12 months of age.
- HBIG (0.06 mL/kg) once shortly after birth.
This regimen has an overall efficacy of 95% [36,37] but the efficacy is lower for mothers with very high HBV viral load [38]. There is no evidence that cesarean section prevents maternal–infant transmission, and thus routine cesarean section is not recommended.
Breastfeeding should be encouraged in women with hepatitis B, irrespective of viral load. While HBV is found in breast milk, transmission by breast milk appears to be rare and the administration of HBV vaccinations and/or immunoglobulin should further decrease any theoretical risks. However, it is our practice to advise temporary suspension of breastfeeding if a woman develops cracked nipples or mastitis.
Acute HBV during pregnancy: general treatment considerations
Treatment of acute hepatitis B infection during pregnancy is primarily supportive. Acute hepatitis often presents with nausea, vomiting, and anorexia so volume depletion is common and may result in decreased uterine blood flow and preterm uterine activity. Hospitalization may be needed to ensure adequate volume resuscitation and to monitor for preterm labor.
In general, no antiviral treatment is indicated as up to 95% of adults will recover spontaneously, but nucleoside analogs (lamivudine, telbivudine or entecavir) are recommended for the 0.5–1.5% of patients who progress to fulminant hepatitis B and those with protracted, severe acute HBV [39].
Maternal exposure to HBV during pregnancy
As with the nonpregnant patient, susceptible patients exposed to HBV should receive HBIG and start the hepatitis B vaccine series. Patients who become pregnant while receiving the vaccine series must be encouraged to complete the series. Due to the common mode of transmission, HIV testing is recommended for all documented hepatitis and all exposures to hepatitis B or C.
Hepatitis C virus
Hepatitis C virus (HCV) has the particular ability to establish chronic infections, leading to cirrhosis and hepatocellular carcinoma; it is the leading cause of liver transplant in North America. Although regarded as a blood-borne infection among adults, mother-to-child transmission is the leading cause of hepatitis C infection among infants and children, and more so if there is maternal HIV-HCV co-infection. Currently, HCV infection is not preventable by vaccine and antiviral therapies have shown only limited response rates.
Virology
Hepatitis C virus is an RNA virus of the Flaviviridae family. There are six recognized genotypes worldwide but each genotype includes thousands of subtypes, also called quasispecies, as a result of its high propensity to mutate. Envelope proteins express hypervariable regions that undergo high mutation rates under the pressure of host antibodies and its RNA polymerase lacks the ability of “self-correction” which among other viral factors account for the HCV’s ability to establish chronic infections and for some of the difficulties faced in developing an effective vaccine.
The overall seroprevalence of HCV infection among pregnant women in Europe and North America ranges from about 0.2% to 4.3%; two-thirds of these women have detectable serum levels of HCV RNA and close to 30% will develop overt liver cirrhosis [40,41]. History of substance abuse appears to be the most common risk factor for chronic HCV infection in pregnancy in Europe and North America [42].
Pathogenesis
Hepatitis C virus infection is most efficiently acquired from parenteral inoculation (i.e. IV drug use or exposure to blood products). Acquisition from percutaneous exposure (medical procedures in resource-poor settings or tattooing) as well as sexual contact has been documented, but is less efficient [42]. Mother-to-child transmission (MTCT) is the most common route of infection among infants and children.
Hepatitis C virus preferentially infects and replicates in hepatocytes but is not directly cytopathic to them. HCV RNA viremia is detected in plasma within days of exposure, but peaks in the first 8–12 weeks of infection and then fluctuates if chronic infection occurs. Liver enzymes (ALT in particular) rise within 4 weeks of infection. Although antibodies are detectable 2–4 weeks following infection, the lack of a vigorous T-lymphocyte response and the virus’s tendency to mutate result in a high rate of chronic infection.
Pathogenesis of MTCT
Most recently reported rates of transmission from infected mothers (mostly chronic infections) range between 3.6% and 6.2% [43,44]. The predominant mechanism has traditionally been thought to be intrapartum (perinatal) but debate exists about whether a greater proportion happens in utero [45]. Breastfeeding is not an established mechanism of transmission despite the fact that HCV can be found in breast milk [46,47].
The risk factors that increase the risk of mother-to-child HCV transmission include HCV viremia (i.e. detectable HCV RNA in maternal blood), maternal HIV-HCV co-infection (which increases transmission rate up to three times) [48], rupture of membranes for greater than 6 hours prior to delivery [44,46], and intrapartum exposure to maternal blood (i.e. perineal lacerations) [49]. Given that level of HCV viral load has inconsistently been reported as an independent risk factor for transmission, it should not be used alone to counsel women on risk of transmission. Neonatal factors have also been implicated in the pathogenesis of MTCT; HLA-DR13-positive neonates seem to be less likely to acquire HCV infection from their mothers, which may be related to a better infant immune response [50].
Clinical presentation
The clinical course and presentation of acute HCV infection in pregnancy do not differ from age-matched nonpregnant adults. Following an incubation period of 7 weeks, acute hepatitis C may cause malaise, nausea, and right upper quadrant pain, followed by dark urine and jaundice. However, more than 75% of acute HCV infections do not develop jaundice and do not have documentation of the laboratory abnormalities typically associated with acute viral hepatitis (i.e. acute ALT increments to >10 times the upper limit of normal). The rate of spontaneous clearance of infection after acute HCV is estimated to be 20–25%. Host factors associated with spontaneous clearance include age <40 years, female sex, and presence of jaundice. Extrahepatic manifestations are not prominent in acute but are seen in chronic HCV infection, and include essential mixed cryoglobulinemia, membranoproliferative glomerulonephritis, and porphyria cutanea tarda. HCV chronic infection leads to hepatic fibrosis; cirrhosis and hepatocellular carcinoma may develop 30–40 years after infection.
Other obstetric considerations
Hepatitis C virus infection during pregnancy is not a recognized cause of congenital malformations, pregnancy loss or stillbirth [51].
Diagnosis
Universal prenatal screening for HCV infection is not recommended at present. Prenatal testing should be guided by evidence of any risk factors for HCV infection including maternal history of intravenous drug use, HIV or HBV infection, solid organ transplant or transfusion recipient before 1992, hemodialysis, piercing and increased transaminases. HCV infection is also found more commonly in women with intrahepatic cholestasis of pregnancy. Anti-HCV antibodies are the test of choice for screening. Positive anti-HCV antibodies do not imply recovery from infection or lack of contagiousness, so they must be followed by serum HCV RNA identification which, if present, defines active infections. HCV viremia is known to fluctuate during the course of chronic infection but one determination during pregnancy is likely to be sufficient for diagnosis and counseling of pregnant women. Transmission of HCV is extremely unlikely from a HCV antibody-positive and HCV RNA-negative mother.
Infants born to HCV-infected mothers should have anti-HCV antibodies done at age >16 months (when maternal antibodies should have disappeared from the infant’s serum) or nucleic acid testing on two occasions between ages 2 and 6 months [47]. Definitive antibody testing of the infant is delayed until >16 months of age because it may take this long for a infant to develop its own antibody response to infection, given that maternal antibodies to HCV may persist in the infant circulation for 2–3 months. Some authors even recommend delaying anti-HCV antibody determination to beyond the age of 18 months as cases of late seroconversion have been reported.
Treatment
Antiviral treatment of HCV infection is contraindicated during pregnancy [52]. The drugs currently available to treat HCV infection include ribavirin, which is a known teratogen, and interferon-alpha.
Prevention of MTCT
Internal fetal monitoring and prolonged labor after rupture of membranes in HCV-infected pregnant women should be avoided [47] but many questions remain regarding management of labor, including the best mode of delivery. No randomized controlled trials of planned cesarean section to decrease MTCT of HCV have been conducted; data from cohort studies (with methodologic weaknesses and hence potential for bias) have not provided evidence that planned cesarean section prevents or reduces the incidence of MTCT of HCV [53]. At the time of writing, active infection with hepatitis C is not considered to be an indication for cesarean delivery or a contraindication to breastfeeding. Currently there is no available vaccine but the higher frequency of spontaneous clearance and shortened duration of reinfection in persons with prior immunity to HCV provide a rationale for vaccine development.
Hepatitis E
Hepatitis E virus (HEV) causes an acute, usually self-limiting hepatitis, common in tropical Asia, Africa and Mexico but rarely seen in industrialized countries. It can lead to fulminant hepatitis, which happens more frequently among pregnant women than any other subgroup. It is a major cause of water-borne epidemics associated with poor sanitary conditions.
Virology
Hepatitis E virus belongs to its own genus (Hepevirus) and family (Hepeviridae). Its RNA genome is enclosed within a capsid that is composed of one or possibly two proteins but many questions remain on its antigenicity.
Epidemiology
It is the most common cause of sporadic and epidemic hepatitis among adults in Asia and the second most important cause among adults (after HBV) in the Middle East and North Africa [54]. Attack rates during outbreaks range from 1% to 15%. Peak incidence occurs between ages 15–40 years.
Pathogenesis
Reported epidemics have been mostly water-borne, especially during rainy season or flooding in endemic areas. Once ingested, the virus first appears in the liver, followed by viremia and subsequent shedding in the stool. Liver injury coincides with ALT elevation and appearance of anti-HEV IgM, which argues for immune-mediated liver injury. The mechanisms behind its aggressive course during pregnancy are not understood [55].
Pathogenesis of MTCT
Transplacental transmission of HEV has been documented by specific HEV IgM in cord blood and virus isolation by PCR, in up to 33% of neonates born to women infected in the third trimester [56], resulting in neonatal massive hepatic necrosis and death. Nonetheless, other infants have recovered from in utero infection [57].
Clinical presentation
The incubation period ranges between 2 and 10 weeks. Clinical presentation varies from asymptomatic infection common among children to anicteric, icteric and fulminant hepatitis. Jaundice, flu-like symptoms, fever, chills, anorexia, nausea, and abdominal pain are usually self-limited and resolve within 4 weeks. ALT is markedly elevated and may precede the onset of symptoms by 10 days. HEV infection does not cause chronic hepatitis, cirrhosis or hepatocellular carcinoma.
Pregnant women during the second and third trimesters are more susceptible to infection by HEV and progression to fulminant hepatic failure [57]. Mortality rates as high as 26.9% have been reported recently [56] in addition to high rates of preterm deliveries. There is no evidence for associated developmental anomalies. Available data suggest that breastfeeding does not increase MTCT [58].
Diagnosis
Specific anti-HEV IgM and IgG can document maternal and neonatal infections. Such testing may only be available in reference laboratories in areas where hepatitis E is not endemic. Molecular techniques for HEV RNA detection in stool and blood are not yet standardized for commercial use. The virus is difficult to grow on cell cultures.
Treatment
Treatment during pregnancy is supportive. There are no data on antiviral therapy for human infections.
Prevention
Postexposure prophylaxis with immunoglobulin does not reduce disease incidence [59].
Autoimmune hepatitis (AIH) is a disorder of unknown etiology that most commonly occurs in women of childbearing age [60]. As AIH is often diagnosed in young women, a considerable number of affected women will want to conceive. Older studies reported reduced fertility rates due to oligomenorrhea secondary to impaired function of the hypothalamopituitary gonadal axis. However, with immunosuppressive treatment, the disease usually improves and menstruation returns.
Diagnosis
Autoimmune hepatitis is classified according to the pattern of autoantibodies that are raised. The different subgroups are associated with different HLA serotypes. HLA-DR3 seropositivity occurs more commonly in early-onset, severe disease, i.e. the form of disease that is most commonly seen in young women.
Epidemiology
Autoimmune hepatitis occurs more commonly in women than men. However, it may affect children and adults of any age and individuals of either gender. The classification of autoimmune hepatitis is shown in Table 9.3. All disease types affect individuals from a variety of ethnic groups. Patients with progressive disease can develop cirrhosis, and hepatocellular carcinoma may also occur.
Table 9.3 Classification of autoimmune hepatitis
| Typical antibodies | Clinical features | |
| Type 1 (classic) | Most commonly: antinuclear antibodies (ANA) anti-smooth muscle antibodies (SMA) anti-actin antibodies May also see: antimitochondrial atypical perinuclear antineutrophilic cytoplasmic antibodies pANCA Antisoluble liver/liver pancreas (anti-SLA/LP) |
|
| Type 2 | Anti-liver kidney microsome 1 antibodies (anti-LKM-1) Anti-liver cytosol-1 antibodies (anti-LC-1) |
|
Etiology/pathophysiology
Autoimmune hepatitis has a complex etiology with genetic and environmental components. HLA-DR3 and -DR4 are the main loci that have been shown to influence susceptibility to the disease. In susceptible individuals, AIH may be triggered by additional environmental events. It has been proposed that the disease can be precipitated by viral infections, e.g. measles, hepatitis, cytomegalovirus and Epstein–Barr virus, or drugs, e.g. methyldopa, diclofenac or nitrofurantoin.
The autoimmune process in AIH is driven by T-cell-mediated responses and T-helper 1 (Th1) cells. Therefore it has been proposed that the shift from a Th1 to Th2-driven immune response in pregnancy may explain the remission of AIH that is often seen in pregnant women.
General course outside pregnancy
The clinical presentation of AIH is variable. Presenting features range from nonspecific symptoms of malaise to fulminant hepatic failure. Common presenting symptoms include fatigue, arthralgia, nausea, anorexia and pruritus. A recent Italian multicenter study reported that older patients more commonly have an acute presentation [61]. There may be signs of liver disease including jaundice, hepatomegaly and splenomegaly. Biochemical abnormalities include raised liver transaminases, bilirubin, and a prolonged prothrombin time. A sizeable proportion of cases will have cirrhosis at the time of diagnosis. However, even in individuals without apparent chronic disease at the time of diagnosis, the liver biopsy often shows features of cirrhosis, consistent with the insidious presentation of AIH. Disease complications include cirrhosis, ascites and esophageal varices and approximately 10% of patients will require liver transplantation within 10 years. In some studies, but not all, this is more likely in patients with cirrhosis at the time of presentation. However, in the majority of patients remission can be induced with the use of immunosuppressive treatment. Affected individuals may also have co-existing autoimmune disease, most commonly type 1 diabetes or thyroid disease.
Clinical presentation in pregnancy
Symptoms
Approximately 6% of AIH cases present in pregnancy [61]. The presenting features are the same as outside pregnancy. One case presented with pre-eclampsia in addition to a de novo diagnosis of AIH [62], indicating the importance of considering other diagnoses when women present with marked hepatic impairment in association with pre-eclampsia. There are two reports of women newly diagnosed in pregnancy who presented with fulminant hepatic failure [62,63].
Signs
Most women with AIH in pregnancy will not have signs of hepatic dysfunction. However, some will have cirrhosis, hepatomegaly and/or splenomegaly.
Laboratory tests
It is important to measure liver function tests and autoantibodies of relevance to liver disease (see Table 9.3) and it may be warranted in some cases to test for those that are found in association with adverse pregnancy outcome (anti-Ro/SSA, anti-La/SSB and anticardiolipin antibodies). In women with abnormal liver function tests, it is important to also measure the prothrombin time and a blood glucose level. A full blood count may reveal thrombocytopenia in women with hypersplenism. Thyroid dysfunction and type 1 diabetes are the most common autoimmune conditions that co-exist with AIH [61] and therefore it is advisable to screen specifically for these disorders.
Diagnostic testing
In addition to laboratory tests, women with AIH should have an abdominal ultrasound to visualise the liver, spleen and, if possible, portal venous circulation. Magnetic resonance imaging (MRI) is often also useful to establish whether a woman with AIH has portosystemic varices.
Differential diagnosis
If a woman with AIH develops hepatic impairment in pregnancy, it is most likely to be caused by a flare in the disease and should be managed as such unless there is evidence to the contrary. The differential diagnosis includes viral infection, a drug reaction, pre-eclampsia or cholestasis.
Management
Preconception counseling
A Swedish survey of 63 pregnancies in 35 women with AIH revealed that 48% did not consult their doctor before becoming pregnant [64]. It is therefore important for hepatologists and fertility specialists to refer affected women for preconception counseling. In particular, women should be advised to continue their immunosuppressive treatment. In addition, they should be screened to establish whether they have gastroesophageal varices as banding should be considered before conception.
Potential fetal complications
Table 9.4 summarizes the rates of adverse fetal events seen in pregnancies where the mother has AIH.
Table 9.4 Fetal outcome in pregnancies complicated by AIH
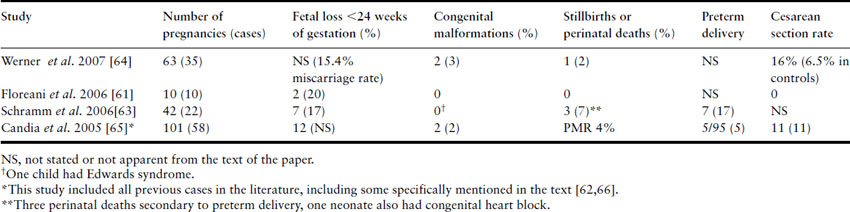
In a retrospective German survey of 42 pregnancies complicated by AIH in four centers, 26% had an adverse pregnancy outcome [63]. The principal causes were spontaneous early fetal loss and preterm labor. The authors identified seropositivity for antibodies to Ro/SSA as a risk factor for adverse pregnancy outcome with an odds ratio of 27 (95% confidence interval (CI) 2–369). In other studies, preterm labor is the main cause of adverse fetal outcomes, including the relatively high perinatal mortality rate. There is no evidence that immunosuppressive therapy with azathioprine is associated with adverse pregnancy outcome [63]. To date, no studies have shown an increased congenital abnormality rate in AIH, regardless of whether women are treated with immunosuppressive agents.
Potential maternal complications
The maternal complications of AIH are summarized in Table 9.5. It is clear that disease flares are relatively common in pregnancy. However, flares during pregnancy are less common in the more recent case series. The oldest study in the table summarized the rate of flares in 101 pregnancies complicated by AIH reported between 1996 and 2004. In this series there were 47 flares, the majority of which (35) occurred prior to delivery and there were 12 in the postpartum period [65]. The more recent studies report lower rates of antepartum flare and a higher frequency of postpartum flares [61,63,64]. Flares in pregnancy may be less common in these studies because women are less likely to stop their immunosuppressive treatment now that there are better safety data for the use of prednisolone, prednisone and azathioprine in pregnancy. Also the diagnosis of AIH was not as certain in the older series, so some cases may have had an exacerbation of a different disease pathology during pregnancy. These data indicate that it is advisable for women with AIH to continue taking glucocorticoids and azathioprine during pregnancy. In the more recent studies postpartum flares are more common. Women should be aware of this and should report symptoms suggestive of a flare as soon as they occur. Doctors caring for women with AIH should also consider increasing the dose of immunosuppressive therapy in the third trimester or immediately after delivery.
Table 9.5 Maternal complications in AIH
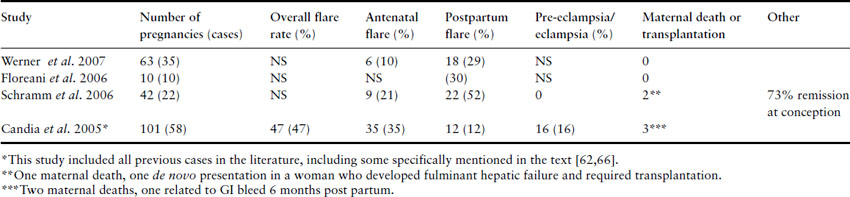
A subgroup of women with AIH have cirrhosis when they conceive. In some series, there is no difference in maternal outcome in women with cirrhosis [64]. Disease activity at the time of conception also does not appear to predict whether a woman will have a flare [63].
In a retrospective German case series that included 42 pregnancies in 22 patients, there was one maternal death and one woman required transplantation following presentation and diagnosis of AIH in pregnancy and subsequent development of fulminant hepatic failure [63]. In a British study of 35 pregnancies in 18 AIH patients, there was one maternal death in a woman who had pulmonary hypertension secondary to microthrombi and another woman died 6 months post partum from a gastroesophageal variceal bleed [62]. Two additional women died within 3 years of pregnancy in the British study.
Antenatal care
Early
Medications
Most women with AIH are treated with prednisolone and azathioprine, although cyclosporine and tacrolimus may also be used. These drugs should be continued, as should other immunosuppressive drugs if they are required to maintain remission of the disease. Flares should be treated with corticosteroids and often also respond to an increased dose of other immunosuppressive medication. Most flares will respond well to medical treatment.
If azathioprine is used for the first time in pregnancy it is important to measure the activity of maternal enzymes that metabolize thiopurines, e.g. thiopurine S-methyltransferase (TPMT), to help identify those woman at most risk for azathioprine-related bone marrow suppression and hepatotoxicity. Women with gastroesophageal varices can be treated with propranolol to reduce the risk of variceal bleeding. Proton pump antagonists or H2 antagonists should also be considered to reduce gastric acid production.
Nonmedications
Women with AIH should be advised to inform the medical team if they have any symptoms suggestive of a flare. Women with portal hypertension should be managed by a multidisciplinary team. They should be educated about the appearance of melena, and if they have any symptoms suggestive of gastrointestinal bleeding they should go immediately to a hospital where massive gastrointestinal hemorrhage is regularly managed.
Close to term
Women should be informed of the risk of having a flare in the postpartum period and consideration should be given to augmentation of immunosuppressive treatment to reduce the chance of a subsequent flare.
Labor and delivery
In some series there is a higher cesarean section rate in women with AIH compared to controls [64]. However, women with uncomplicated disease who have not had a flare should be able to have a normal labor and delivery. If a woman with AIH has portal hypertension, it is advisable to avoid pushing in the second stage of labor. However, providing there are no abnormalities of clotting or platelets, it is feasible to have a normal delivery with an assisted second stage.
Postpartum period
Adjustments to treatment
If immunosuppressive therapy has been increased at the end of pregnancy, this should be maintained for 4–6 months after delivery. Women with AIH should be followed by a hepatologist at this time and treatment should subsequently be adjusted according to disease severity.
Long-term health concerns
Most women with AIH are treated effectively with immunosuppressive therapy. However, the condition can be complicated by progressive liver damage, cirrhosis and hepatic failure. There is no evidence that pregnancy worsens the course of AIH.
Advice for subsequent pregnancies
There are no studies that indicate that the prognosis worsens (or improves) in subsequent pregnancies. If a woman with AIH wants a subsequent pregnancy, it is advisable to conceive whilst in remission.
Issues related to medications and lactation
Azathioprine and prednisolone/prednisone enter breast milk in low concentrations. Given the potential maternal risk from stopping treatment, women should continue taking their medication and may be reassured that there are no data documenting adverse fetal effects from exposure to thiopurine metabolites in breast milk.
Contraceptive recommendations
Providing they have well-controlled disease, women with AIH can use any form of contraception. If hepatic impairment is present, estrogen-containing contraceptives are usually discouraged.
Conclusions
Women with AIH can have disease flares in pregnancy and in the puerperium, and therefore should be discouraged from stopping immunosuppressive treatment. Indeed, there is an argument for increasing their treatment dose close to term.
Intrahepatic cholestasis of pregnancy
Intrahepatic cholestasis of pregnancy (ICP), also known as obstetric cholestasis, most commonly presents in the third trimester with maternal pruritus and liver dysfunction. It causes transient maternal cholestasis that resolves after delivery and is associated with fetal complications, including spontaneous preterm labor and in rare cases third-trimester intrauterine death.
Epidemiology
Intrahepatic cholestasis of pregnancy affects 0.5–1.5% of pregnancies in Europeans and is more common in women of South Asian and South American origin. Most affected women are asymptomatic outside pregnancy, although up to 20% of cases have pruritus in the second half of the menstrual cycle or if they take estrogen-containing contraceptives.
Etiology/pathophysiology
The condition has a complex etiology with genetic and endocrine components. Evidence for genetic factors includes sibling studies that report a 20-fold increase in risk for first-degree relatives, pedigree studies that demonstrate sex-limited autosomal dominant inheritance in a subgroup of cases and reports of heterozygous mutations in genes that encode biliary transporters or receptors. It is likely that these genetic abnormalities cause women to be predisposed to the cholestatic effects of reproductive hormones.
Raised serum levels of reproductive hormones are thought to play a role in the etiology of ICP because the condition is more common in twin pregnancies in which estrogen levels are higher, and because women with a history of cholestasis may develop pruritus and abnormal liver function when given exogenous estrogens. The condition also occurs more commonly following the administration of progesterone to prevent preterm labor. There is also accumulating evidence that reproductive hormones and/or their metabolites influence the expression and function of hepatic bile acid transporters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree