Disorders of Amino Acid Transport Across Cell Membranes
Kirsti Näntö-Salonen and Ollie Simell 
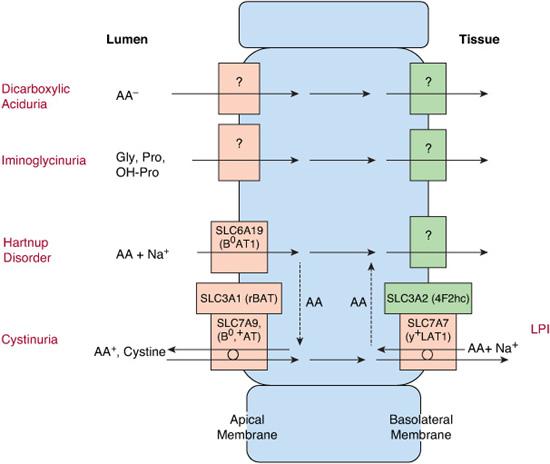
Inherited defects in amino acid transport at the cell membrane (Fig. 143-1) are expressed as selective renal aminoaciduria and impaired intestinal absorption. Their symptoms result from excess of certain amino acids in the urine or lack of them in the tissues. Consequently, in cystinuria, renal stones are formed because of high urinary concentration of poorly soluble cystine. In lysinuric protein intolerance, lack of the urea cycle intermediates arginine and ornithine leads to hyperammonemia and protein intolerance. The pellagra-like dermatitis and ataxia in Hartnup disorder are attributed to deficiency of tryptophan, the precursor of niacin synthesis (Fig. 143-1).1-3
CYSTINURIA
Cystinuria (OMIM 220100; OMIM 600918) causes a lifelong risk of urolithiasis.4-9 Its average incidence is 1:7000 but varies considerably between different populations. A defect of the high-affinity luminal transporter for cystine and dibasic amino acids in the epithelial cells in the jejunal mucosa and in the proximal renal tubulus leads to poor intestinal absorption and poor reabsorption of these amino acids in the kidney. If the intratubular cystine concentration exceeds the threshold of solubility, crystals and stones will form. 
Cystinuria has been classified into two subtypes: type I is the pure autosomal recessive form of the disease that represents over 60% of the cases, and non-type 1 is inherited in a dominant mode with incomplete penetrance. 
Type I cystinuria is caused by at least 103 mutations in the SLC3A1 gene on chromosome 2p. Non-type I cystinuria is caused by at least 66 mutations in the SLC7A9 gene on chromosome 19q.8 These genes encode the heavy and the light subunits of the amino acid transporter, respectively. A new genetics-based classification has been suggested: type A for SLC3A1 homozygotes, type B for SLC7A9 homozygotes, and type AB for the mixed type.9
Some patients never develop kidney stones, but others have recurrent symptoms from early childhood, with acute episodes of abdominal or lower-back pain, hematuria, pyuria, or passing of stones. 
Cystine stones are usually radio-opaque and visible on ultrasonography. They are often located in the bladder. A positive urinary nitroprusside test and analysis of urinary amino acids lead to the diagnosis. Plasma concentrations of cystine and the dibasic amino acids are normal or slightly decreased. Excessive hydration to dilute the urine and alkalinization, preferably with potassium citrate, to improve cystine solubility are the cornerstones of therapy. Restriction of dietary animal protein to limit endogenous cystine synthesis from methionine may be helpful, and moderate sodium restriction also decreases cystine excretion.7,19,20
If the standard therapy fails a thiol derivative, D-penicillamine or mercaptopropionylglycine (tiopronin), is added to decrease urinary free cystine concentration by forming water-soluble compounds and by cleaving the disulfide bond of cystine to more soluble cysteine.7,19,20 Captopril is well tolerated but may not be as effective as the other thiol compounds.7,19,20 New, minimally invasive urological techniques minimize the need for open surgery for stone removal. Recurrent urinary tract infections, urinary obstruction, and renal insufficiency are possible complications. 
Regular follow-up is essential to support treatment compliance, to monitor renal function, and to detect developing stones early. 
LYSINURIC PROTEIN INTOLERANCE (LPI)
 METABOLIC DERANGEMENT AND PATHOPHYSIOLOGY
METABOLIC DERANGEMENT AND PATHOPHYSIOLOGY
Hyperammonemia after protein ingestion and protective aversion to high-protein foods make lysinuric protein intolerance (LPI; OMIM 222700) resemble urea cycle enzyme deficiencies (see Chapter 145). Only 130 patients have been reported, with the highest incidence in Finland.27 The transport of the dibasic cationic amino acids lysine, arginine, and ornithine is defective at the basolateral membranes of epithelial cells in the renal tubules and small intestine.28-31 Limited intestinal absorption of these amino acids and massive urinary loss of especially lysine result in low plasma concentrations. 
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


