Diarrhea
Ricardo A. Caicedo and Ivor D. Hill
Diarrhea is an increase in the liquidity and/or frequency of the stools. It reflects an increase in stool water content due to impaired water absorption and/or active water secretion by the intestine. Most episodes of diarrhea occur on the basis of 1 of 5 mechanisms: malabsorptive, secretory, osmotic, dysmotility, and inflammatory.  One or more of these mechanisms may be operative in an individual during an episode of diarrhea.
One or more of these mechanisms may be operative in an individual during an episode of diarrhea.
ACUTE DIARRHEA
Acute diarrhea accounts for 2 to 3 million deaths per year with most occurring in young children in developing countries. In the United States, approximately 220,000 children under 5 years of age are hospitalized each year for acute diarrheal illnesses, accounting for 9% of all hospitalizations in this age group.2
 CLINICAL FEATURES AND DIFFERENTIAL DIAGNOSIS
CLINICAL FEATURES AND DIFFERENTIAL DIAGNOSIS
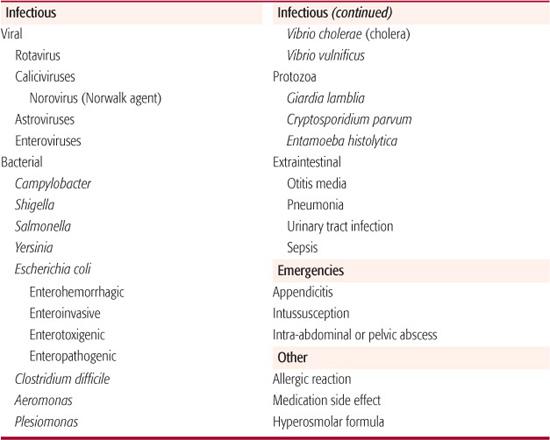
The common causes of acute diarrhea are listed in Table 385-1. Enteric infections account for most cases of acute diarrhea in children.3 Detailed information regarding each specific pathogen is found in Section 17. Viruses are responsible for the majority (60–80%) of enteric infections in children, especially those under the age of 2 years. Of these, rotaviruses are most prevalent and most likely to cause dehydrating diarrhea. Of all children requiring treatment for dehydration during the peak winter gastroenteritis season, 70% to 90% have rotavirus infection.4 Other winter-predominant viral agents, such as astroviruses and caliciviruses, tend to infect older children and cause illness of shorter duration. The norovirus (or Norwalk agent), a calicivirus, is an important cause of foodborne outbreaks of gastroenteritis in schools, day-care centers, hospitals, cruise ships, and among athletic teams. Enteroviruses tend to peak during the summer months and cause diarrhea that is more prolonged than that induced by rotaviruses.
Bacterial pathogens commonly associated with diarrhea include Campylobacter, Shigella, Salmonella, Yersinia enterocolitica, and various types of Escherichia coli. Campylobacter jejuni is the most common bacterial cause of acute gastroenteritis in developed countries. Enterohemorrhagic E coli, including the prototypical strain O157:H7, elaborate Shiga-like toxins that predispose to hemolytic uremic syndrome, typically developing 2 to 14 days after onset of diarrhea. Clostridium difficile causes a disease spectrum ranging from mild diarrhea to fulminant pseudomembranous colitis.6 Dysentery, characterized by the presence of blood, pus, and mucus in the stools, usually occurs with bacterial causes of diarrhea. Non-dysentery bacterial diarrheas are caused by Aeromonas, Plesiomonas, and Vibrio cholerae.
Protozoal enteric infections can manifest either as acute or persistent diarrhea. Acute giardiasis is marked by sudden onset of profuse watery diarrhea, abdominal cramps, and bloating. Cryptosporidium parvum typically causes large-volume watery diarrhea, nausea, vomiting, and fever. It has been associated with communitywide outbreaks and is a relatively common cause of diarrhea in immuno-compromised individuals such as those with AIDS.
 DIAGNOSTIC EVALUATION
DIAGNOSTIC EVALUATION
Most cases of acute infectious diarrhea are self-limiting and resolve completely in 5 to 7 days. Management of these cases should therefore be aimed largely at maintaining hydration with oral rehydration therapy and promoting continued nutrition while avoiding unnecessary diagnostic investigations and the inappropriate use of medications. In some cases, diarrhea is indicative of a more serious underlying condition. Early diagnosis and treatment of these cases is essential in order to minimize the potential for serious morbidity and mortality. Young children with intestinal intussusception often present with bloody mucoid diarrhea associated with severe cramping abdominal pain, inconsolable crying, and, in some cases, lethargy. Acute appendicitis can present initially with diarrhea prior to onset of the classical symptoms of abdominal pain. In a retrospective series of 63 children under 3 years of age with appendicitis, one third had diarrhea as the initial symptom.7 The inflamed appendix irritates the colon and produces frequent small-volume mucous-containing stools. This same process can occur in the case of an intraabdominal or pelvic abscess. C difficile–induced pseudomembranous colitis and shigellosis can progress to toxic megacolon, which can lead to perforation, peritonitis, systemic sepsis syndrome, and shock. Infections with Shiga-like toxin–producing organisms such as enterohemorrhagic E coli often present with bloody diarrhea and can progress to the hemolytic uremic syndrome. This potentially fatal disease is characterized by the triad of hemolytic anemia, thrombocytopenia, and acute renal insufficiency.
Table 385-1. Common Causes of Acute Diarrhea in Infants and Children
History In children presenting with acute diarrhea, the history should focus on timing and onset, frequency and volume of stool output, and stool characteristics. Watery diarrhea suggests more proximal small intestinal disease, while diarrhea containing blood and mucus usually implies colonic inflammation. Vomiting suggests the presence of an organism that infects the upper intestine, such as enteric viruses, enterotoxin-producing bacteria or giardian. Pain in the lower abdomen and rectum or pain with defecation (tenesmus) indicates distal colonic involvement as seen with bacterial infections. A history of childcare center attendance; similar sick contact exposure; travel to a diarrhea-endemic area; recent use of antimicrobial drugs; and ingestion of seafood, unwashed vegetables, unpasteurized milk, contaminated water, or uncooked meats provide valuable clues as to the possible cause of the diarrhea. Institutionalized children and those recently returning from developing countries are more likely to have bacterial or parasitic pathogens.
Additional information obtained on history should be aimed at determining the severity of the stool fluid losses and identifying other conditions that could potentially place the child at added risk for an adverse outcome. Thirst is an early symptom of impending dehydration. The amount of oral intake of fluids and voiding frequency should be determined as part of the history. Fever is a component of the inflammatory response to infection but may also occur as a result of dehydration. Increased respiratory rate or work of breathing may indicate acidosis, while lethargy and a decrease in sensorium are features of shock. Information about an underlying chronic disease or immunodeficient state is important, as is a history of prior intestinal surgery. Diarrhea in the child with a chronic illness or immunodeficiency requires more extensive evaluation because of the additional risk for opportunistic infections. Very young infants are at increased risk for complications of diarrheal illnesses. Dehydration occurs more rapidly in this age group, and those under 2 months of age with bacterial infections causing diarrhea are at increased risk for an associated bacterial septicemia.8
Physical Examination In children with acute diarrhea examination should be focused on identifying clinical signs of dehydration. Loss of skin tissue turgor and a decrease in mucous membrane moisture are clinical indicators of dehydration. With mild dehydration (0–5%), the mucus membranes are slightly dry and skin tissue turgor is slightly decreased. As dehydration progresses to a moderate degree (5–10%), the mucus membranes will be obviously dry and the decrease in tissue turgor is easily elicited. Dehydration that progresses beyond this stage (≥ 10%) is accompanied by additional features of sunken eyes and a sunken fontanelle in the young infant. Further unreplaced fluid losses will result in hypovolemic shock. 
Laboratory Tests Laboratory tests are seldom required in children with acute diarrhea in the absence of clinical features of dehydration or other worrisome findings such as blood in the stools. The decision to perform any investigations and the choice of test will depend on the clinical concern. Analysis of a urine sample for specific gravity, blood, leukocytes, and culture provides information on the child’s hydration status and may identify a urinary tract infection as the cause of the diarrhea. Blood samples for determining electrolytes, carbon dioxide, and urea nitrogen are sometimes needed to evaluate for electrolyte imbalances, acidosis, and acute renal insufficiency. A complete blood count with differential and platelet count may help in evaluating for sepsis or the hemolytic uremic syndrome. In cases of dysentery or suspected bacterial causes of diarrhea, stool samples should be sent for microscopy and culture. Fecal leukocytes indicate the presence of inflammation in response to invasive or cytotoxin-secreting pathogens. A stool culture should be performed in every child with bloody diarrhea because bacterial pathogens have been implicated in up to 20% of such cases (see Chapter 387).9 Stool rotavirus assays using immunologic or polymerase chain reaction techniques are widely available for the diagnosis of this common cause of acute gastroenteritis. Testing for rotavirus is generally not necessary in cases of suspected viral diarrheas because it is unlikely to have any impact on the management of the child.
 TREATMENT
TREATMENT
Treatment of children with acute diarrhea should first be directed toward preventing dehydration and maintaining nutrition. Use of oral rehydration therapy (ORT) provides rapid, safe, effective, and inexpensive therapy for diarrheal disease. Oral rehydration solutions are effective for treating children regardless of the cause of diarrhea or the child’s serum sodium level at the onset of therapy. Most replacement solutions contain 50 to 90 mmol/L of sodium and 20 to 30 mmol/L of potassium. The addition of glucose (optimally 110–140 mmol/L or 20–25 g/L) enhances the rate of water uptake by promoting transport of sodium across the enterocyte via the brush border membrane glucose-sodium cotransporter.10 Liquids such as fruit juices and sodas have high levels of sugars but contain insufficient electrolytes to replace fecal losses.11 The glucose- and electrolyte-containing solutions ORT should be offered in addition to the regular breast milk, formula, or solid feeds. Vomiting is frequently present during the early stages of an acute viral diarrhea illness but is not a contraindication to the use of oral fluid therapy. Provision of oral fluids in small volumes at frequent intervals is generally well tolerated and minimizes the potential for vomiting. 
For children who present with clinical signs of dehydration, therapy should be aimed at first correcting the fluid deficit and then maintaining hydration by replacing ongoing losses while maintaining nutrition. Correction of fluid deficits can usually be accomplished via oral administration of appropriate glucose electrolyte solutions. Intravenous fluid therapy is required in only a small number of cases in which there is an accompanying ileus or the vomiting is of such magnitude that oral therapy fails. The fluid deficit is calculated on the basis of the clinical degree of dehydration and should provide 50 ml/kg body weight for mild dehydration (5%) and 100 ml/kg body weight for moderate dehydration (10%). The calculated deficit volume should be replaced over a period of 4 to 6 hours by offering frequent small volumes from a bottle, cup, or spoon, or it can be delivered as a continuous slow-rate infusion via a nasogastric tube. This process can be repeated if the dehydration is not fully corrected at the end of the 6-hour period. Once the child is fully rehydrated, the process of providing fluids to maintain hydration should begin, and the child should be encouraged to resume regular feedings.
Children with moderate to severe dehydration who also have clinical findings of shock require urgent therapy to reestablish an adequate circulating blood volume. Fluid resuscitation in these cases requires intravenous administration of an isotonic crystalline solution such as normal saline. If intravenous access is difficult to establish, fluid resuscitation can be achieved by the intraosseous route (see Chapter 107). An initial rapid infusion of 20 ml/kg body weight of normal saline will correct the shock in most children with dehydration due to diarrhea. In those with persistent clinical signs of shock, a second infusion of 20 ml/kg body weight can be administered. Once the child’s circulatory compromise has been corrected, the process of rehydration with maintenance therapy and early reintroduction of feeds as outlined previously should follow.
Antibiotics are generally not recommended for children presenting with acute bloody diarrhea unless a specific pathogen has been isolated. Antibiotic therapy has been implicated as a risk factor for the subsequent development of hemolytic uremic syndrome in patients with diarrhea due to enterohemorrhagic E coli. Confirmed shigellosis and cholera should be treated with an antibacterial such as trimethoprim-sulfamethoxazole. Antibiotics are indicated in immunocompromised children or those with septicemia due to Salmonella, Campylobacter, or Yersinia.13 Some probiotic supplements have been shown to decrease the duration of the acute diarrheal illnesses.14Saccharomyces boulardii, a nonpathogenic yeast, has been used to treat and decrease the recurrence rate of C difficile enterocolitis, and Lactobacillus rhamnosus GG may lessen the severity of rotaviral dehydration.15 Zinc supplementation may be beneficial; it has been shown to reduce the duration and severity of acute diarrhea.16
Antidiarrheal agents are not recommended in the management of acute diarrhea in infants and children. Adsorbents such as magnesium aluminum silicate and psyllium fiber may alter stool consistency but do not reduce absolute fecal water and electrolyte loss. Opiate antimotility agents such as loperamide and diphenoxylate-atropine can cause ileus, nausea, and sedation.
Table 385-2. Differential Diagnosis of Chronic Diarrhea in Infants and Children
CHRONIC DIARRHEA
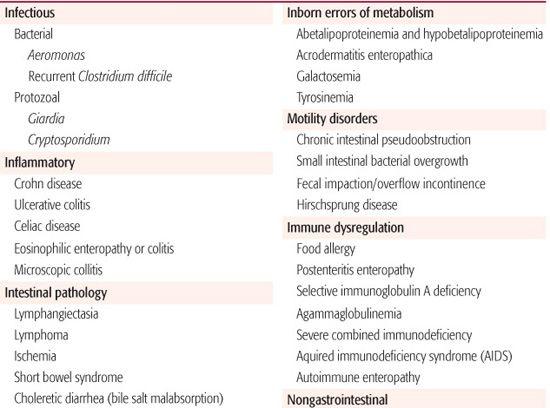
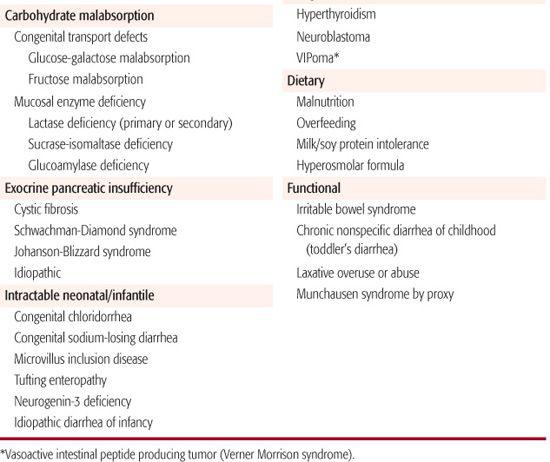
Chronic diarrhea is defined as diarrhea that persists for a period of 2 weeks or longer. In general, the approach to the child with chronic diarrhea involves first trying to determine the primary underlying mechanism (malabsorptive, secretory, osmotic, inflammatory, dysmotility) and then the specific cause. The differential diagnosis of chronic diarrhea in children is shown in Table 385-2. Diarrhea may be benign with no risk for any complications (eg, chronic, nonspecific diarrhea of childhood) or may be indicative of a serious underlying condition. Infants and young children with chronic diarrhea and failure to thrive and those with secretory diarrhea (ie, continues during fasting) must be evaluated with more urgency because of a potential for significant morbidity.
 CLINICAL FEATURES AND DIFFERENTIAL DIAGNOSIS
CLINICAL FEATURES AND DIFFERENTIAL DIAGNOSIS
Infectious causes of chronic diarrhea include certain bacteria (Aeromonas, Salmonella, Clostridium spp) and protozoans. Giardiasis more commonly presents with diarrhea and abdominal pain lasting weeks to months. Cryptosporidiosis damages the mucosal brush border and can cause malabsorption due to both loss of intestinal disaccharidases and decrease in the absorptive surface area. Many enteric bacterial pathogens also damage the brush border membrane, causing secondary disaccharidase deficiency with subsequent carbohydrate malabsorption and chronic diarrhea (see Chapter 408). Autoimmune inflammatory processes such as celiac disease, Crohn disease, ulcerative colitis, and autoimmune enteropathy can all present with chronic diarrhea and signs of malabsorption. Chronic, nonspecific diarrhea of childhood, also called toddler’s diarrhea, is a common condition attributed to mild carbohydrate malabsorption and hypermotility.17 Other diet-related causes include food allergy, infant overfeeding, and use of a hypertonic formula. Malabsorption of fat is seen in cystic fibrosis and other conditions of exocrine pancreatic insufficiency as well as in cholestatic liver disease and intestinal lymphangiectasia.
Immunodeficiency states can present with chronic diarrhea (see Chapter 391). Idiopathic diarrhea of infancy remains a potentially fatal condition, as do the congenital transport defects and inborn errors of metabolism. Conditions such as functional constipation and Hirsch-sprung disease leading to recurrent fecal impaction provoke overflow incontinence, which frequently mimics diarrhea, although enterocolitis can occur with Hirschsprung disease. Colonic motility disorders and postoperative complications such as short bowel syndrome and small intestinal bacterial overgrowth often present with chronic diarrhea (see Chapter 407).
Extraintestinal disorders can also present with chronic diarrhea in infants and children. Examples include hyperthyroidism and catecholamine-secreting tumors.
 DIAGNOSTIC EVALUATION
DIAGNOSTIC EVALUATION
History The age of onset of the diarrhea, duration of diarrhea, and number, volume, and character of stools provide important clues. Symptoms starting shortly after birth suggest a congenital disorder. Diarrhea that persists in a fasting patient is likely due to a secretory process. Small-volume, frequent stools; tenesmus; and urgency suggest inflammation in the distal colon. Large-volume, less frequent stools suggest small bowel or proximal colonic disease. Nocturnal diarrhea and incontinence are concerning symptoms for inflammatory disorders. Explosive, sweet-smelling stools suggest carbohydrate malabsorption, while rancid or foul-smelling, greasy stools suggest fat malabsorption. The presence of blood suggests infectious or inflammatory conditions of the colon, and mucus may be seen in individuals with colitis, irritable bowel syndrome, or allergy. Undigested food particles in stools are typical of toddler’s diarrhea.
Assessment of growth velocity is important. Poor growth in infants or toddlers or weight loss in the older child or adolescent indicates a malabsorptive disorder. The duration of the growth failure may help differentiate a congenital from an acquired malabsorptive syndrome. Congenital disorders and cystic fibrosis usually result in poor growth from the newborn period. In contrast, individuals with celiac disease gain weight normally until the introduction of gluten-containing cereals at about 4 to 6 months of age. Chronic diarrhea with failure to thrive can be a presentation of Hirschsprung disease. A decrease in linear growth velocity is sometimes the initial sign of Crohn disease and may precede the onset of diarrhea by some years.
The presence of associated symptoms and signs also provides valuable clues to the diagnosis. Infants with dietary protein intolerance may have vomiting, skin rashes, and wheezing in addition to diarrhea. Oral aphthous ulcers, arthritis or arthralgia, uveitis, erythema nodosum, and pyoderma gangrenosum are all features associated with autoimmune disorders such as Crohn disease. Other risk factors for infections should be sought, including travel to areas endemic for diarrheal diseases or residence in areas known to be associated with tropical sprue. Well-water ingestion is a risk factor for giardiasis among other infections.19 Immune deficiency states may be suggested by a history of recurrent respiratory, skin, or ear infections. A family history of inflammatory bowel disease or early colon cancer increases a child’s risk of inflammatory bowel disease. A thorough review of the child’s dietary history is essential because it may indicate toddler’s diarrhea, lactose maldigestion, food allergy, and irritable bowel syndrome. A history of laxative overuse should prompt consideration of factitious diarrhea or Munchausen syndrome by proxy.
Physical Exam Examination should include assessment of growth velocities, subcutaneous fat and muscle mass, and abdominal and rectal examination. Decreased subcutaneous tissue or muscle mass, a smooth tongue, dry and cracked lips, decreased deep tendon reflexes, and sparse hair all suggest nutrient deficiencies. Signs of malabsorption include abdominal distension, abnormal bowel sounds, and peripheral wasting. A distended abdomen that is tympanitic suggests colonic gas and carbohydrate malabsorption, whereas one with palpable masses suggests fecal impaction. Perianal fissures, skin tags, and fistulae should raise suspicion of Crohn disease.
Laboratory Tests The absence of evidence for malabsorption, infection, bleeding, or protein loss narrows the differential diagnosis. Infectious etiologies of chronic diarrhea can be identified by stool culture (eg, Campylobacter, Shigella, Salmonella, Yersinia), toxin assay (eg, Clostridium), microscopic examination of stool for ova and parasites, and antigen detection (eg, Giardia, Cryptosporidium). Inflammatory conditions can be screened for by examining stools for leukocytes, occult blood, and fecal α1-antitrypsin assay. Fecal calprotectin (a neutrophil product) and lactoferrin (an acute-phase reactant) are newer markers for gastrointestinal inflammation that are now available.20 Screening for malabsorptive disorders can be accomplished by measuring stool pH, reducing substance, fecal elastase and fecal fat balance. The evaluation of malabsorptive disorders is further discussed in Chapter 408. Fecal α1-antitrypsin is a marker for protein-losing enteropathy, which can be seen in celiac disease, inflammatory bowel disease, infectious enterocolitis, and intestinal lymphangiectasia.
Determination of stool electrolytes and osmolality is used to distinguish osmotic from secretory diarrhea. The osmotic gap represents the difference between normal stool osmolality (290 mmol/L) and twice the sum of stool sodium and potassium concentrations. A normal osmotic gap is less than 125, and usually less than 50. A stool sodium concentration greater than 90 mmol/L and osmotic gap less than 50 indicates a secretory process, whereas a stool sodium less than 60 and osmotic gap greater than 125 suggests an osmotic diarrhea. Some children with chronic diarrhea will have intermediate values for stool sodium and osmotic gap, indicating the presence of both osmotic and secretory components.
The complete blood count with differential is useful to determine the presence of leukocytosis, elevated or suppressed neutrophil counts, and anemia. Anemia may be a sign of chronic inflammation or malabsorption, such as is seen with celiac and Crohn diseases. An elevated erythrocyte sedimentation rate or C-reactive protein suggests an inflammatory process. Quantitative immunoglobulin measurements may identify immunodeficiency in infants with chronic diarrhea and in children with recurrent Giardia or Cryptosporidium infections. Low cholesterol and triglyceride levels are indicative of abetalipoproteinemia. Serologic tests to screen patients for celiac disease are an important consideration in any child with chromic diarrhea.
Additional laboratory tests are dependent on the clinical suspicion and might include sweat chloride for cystic fibrosis, skin-prick testing for food allergy, and various breath tests to evaluate for malabsorption states (see Chapter 408). Upper gastrointestinal barium studies with small bowel follow-through may help identify an anatomic defect or inflammatory condition. A barium enema may be used to determine the position of the cecum (to rule out malrotation), to visualize the anatomy proximal to a stricture, or to look for Hirschsprung disease.
Indications for colonoscopy in children with chronic diarrhea include suspicion of infectious or inflammatory colitis. Indications for upper gastrointestinal endoscopy in children with chronic diarrhea include suspicion of celiac disease, disaccharidase deficiency, intestinal lymphangiectasia, and Crohn disease. Duodenal intubation for secretin-stimulated pancreatic function testing can be done endoscopically or by means of a nasoduodenal tube. Anorectal manometry and suction rectal biopsy are performed to exclude Hirschsprung disease.
 TREATMENT
TREATMENT
The objective in evaluating children with chronic diarrhea is to identify specific treatable conditions and institute appropriate therapy. Examples include the gluten-free diet for celiac disease, anti-inflammatory and immunosuppressant medications for Crohn disease, pancreatic enzyme supplementation for cystic fibrosis, and surgery for Hirschsprung disease. It is just as important to identify those causes of chronic diarrhea that are benign and require minimal diagnostic workup and largely supportive therapy. Chronic nonspecific diarrhea of childhood (toddler’s diarrhea) is characterized by the presence of loose stools in a child with normal growth and who is otherwise well. Reduction in fruit juice intake, especially apple juice that has a high fructose-to-glucose ratio,17 reduction of sorbitol intake, or an increase in dietary fat intake may resolve the problem.
Nutritional rehabilitation is an early essential component of the treatment of children with chronic diarrhea who are malnourished. Inadequate nutrient intake in children with chronic diarrhea often is iatrogenic. Prolonged use of oral rehydration solutions, clear liquids, and elimination diets may result in significant weight loss and nutritional deficits.21 As for children with acute diarrhea, antidiarrheal agents are not recommended for those with chronic diarrhea.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


