Fig. 9.1
Vagino-cervico- hysteroscopy provides a minimally traumatic performance of diagnostic hysteroscopy. It only requires a hysteroscope and a watery distension medium. Steps: (a) hysteroscope locates the cervix with visualisation of ostium externum cervici; atraumatic insertion of the small hysterosope: the distension medium dilates the cervical channel; (b) visualisation of the direction of the cervical channel with further insertion into the uterine cavity; after insertion, the hysteroscope is rotated about the axis for visualisation of the uterine cavity (c)
Requirements for Minimally Invasive Hysteroscopy
Ambulatory endoscopic unit
Small diameter instrumentation with high optical quality
watery distension medium
low intra-uterine pressure
atraumatic technique (vagino-cervico-hysteroscopy)
Hysteroscopy, Laparoscopy, and Indirect Imaging
Although hysteroscopy provides direct visualisation of the uterine cavity, a major drawback is that it is difficult to make exact measurements of intra-uterine pathology, and more specifically, to measure the indentations of the uterine fundus. These measurements are based on subjective estimations performed at the time of examination. It is therefore not surprising that, in a recent report, the international inter-observer agreement was very disappointing for hysteroscopic distinctions between a septate and arcuate uterus (ICC 0.27) [15, 16]. However, a recent study showed that the accuracy in detecting intra-uterine pathology with hysteroscopy was higher than with HSG; the reported agreement between the two procedures was only 33.3 % in the diagnosis of uterine septum/subseptum [17, 18]. Like the HSG, the hysteroscopy does not allow visualisation of the outer uterine contours. For an accurate diagnosis, supplementary examinations with ultrasound and laparoscopy are necessary.
Previously, hysteroscopy and laparoscopy were the gold standard for diagnosing and evaluating congenital uterine malformations [19, 20]. However, endoscopic diagnosis relies on the surgeon’s subjective impressions and lacks strict objective criteria and measurements; thus, it does not allow assessments of subtle uterine morphological differences [21]. The AFS classification system, which is used routinely, does not include morphological criteria. With the inability to perform exact measurements, it is not surprising that there is wide variability in estimations of the prevalence of uterine anomalies among different studies, and more specifically, in the diagnoses of septate and arcuate uteri. Without a means for making accurate measurements and standardised procedures for performing these measurements, it will not be possible to determine the true incidence of uterine congenital anomalies and their impact on fertility and reproductive outcome.
The 3-D ultrasound approach offers a promising means for making exact measurements of morphological alterations in congenital uterine pathology (Fig. 9.2). A study by Salim et al. [22] reported very good inter-observer agreement with 3-D ultrasound measurements. They demonstrated the feasibility of performing studies to investigate the reproducibility of diagnoses of uterine anomalies. Once exact measurement techniques are standardised, it will be possible to make comparisons among data from different studies.
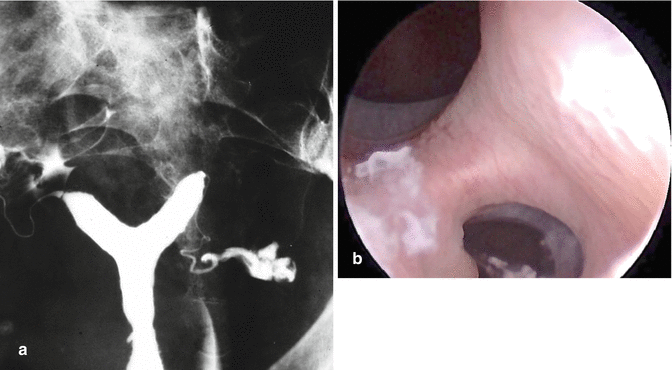

Fig. 9.2
Like the HSG, (a) hysteroscopy (b) shows a clear division in the uterine cavity. A 3D ultrasound examination is required to make a final differential diagnosis between a partial septate uterus (U2a) or a partial bicorporeal uterus with (U3c) or without a partial septum (U3a)
In a recent publication, Ludwin et al. [23] compared the accuracy of 2-D and 3-D ultrasound to the gold standards of hysteroscopy and laparoscopy. They demonstrated accuracies of 100 % for 3-D SIS, 97.4 % for 3-D, 94 % for 2-D SIS, and 90.6 % for 2-D, when performed by experts. Several other studies have also mentioned high accuracy rates for 3-D ultrasound in the detection of uterine anomalies compared to hysteroscopy and laparoscopy [24–26]; the best results showed 100 % sensitivity, specificity, and accuracy.
There is growing evidence that 3-D ultrasound may replace hysteroscopy and laparoscopy as the gold standard for the diagnosis and classification of aberrant uterine morphology; particularly for non-complex uterine anomalies, like classes U1, U2, and U3, according to the new classification system of Grimbizis et al. [27, 28]. Although hysteroscopy is currently considered a minimally invasive procedure, it requires training, and the risk of complications remains relevant [29].
In the diagnosis of more complex anomalies, hysteroscopy and laparoscopy continue to play important roles. In adolescents with severe dysmenorrhoea, a complete exploration should be performed, starting with a careful examination of the vagina. A visualisation of the cervix should be performed by direct visual inspection or by vaginoscopy, and it is necessary to exclude cervico-vaginal aplasia. Additional information can be gained with indirect visualisation methods, like 3-D ultrasound and magnetic resonance imaging (MRI). Indirect imaging should be conducted for identifying the presence, localisation, and size of haematometra, haematocolpos or pyocolpos. MRI is typically reserved for complex or indeterminate cases, it is non-invasive and allows excellent soft tissue visualisation [30]. Non-descended ovaries are well known to occur in case of uterine anomalies [31]; due to difficulties in visualising the key regions, this condition can be missed with laparoscopy. MRI can be useful for locating these ovaries. Some authors advise performing ovarian stimulation with clomiphene to improve and facilitate visualisation of these ovaries during MRI [32].
Laparoscopy can provide the means for exact descriptions of aberrant uterine anatomy, it can detect the partial presence or absence of tubes and ovaries, and it can determine normal or abnormal positioning. Laparoscopy is also necessary for a differential diagnosis of uterine malformations, like a non-communicating rudimentary horn or juvenile cystic adenomyoma [33].
Many patients experience problems with infertility or recurrent pregnancy losses; thus, concomitant pathology that might interfere with fertility must be excluded. Among cases of congenital uterine anomalies, endometriosis occurred in 20–30 % of patients [8, 34–37]. Among cases with obstructive pathology, endometriosis occurred in 77 % of patients [38]. Laparoscopy offers the potential for both diagnosing and surgically treating pathology, when indicated.
Laparoscopy provides direct visualisation of the pelvis and facilitates the identification of congenital uterine anomalies. However, it requires the aid of indirect imaging techniques to determine whether a rudimentary cavity is present in cases with a rudimentary horn (Fig. 9.3).
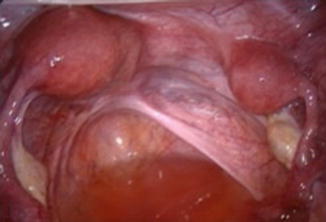

Fig. 9.3
Laparoscopic visualisation of a hemi uterus (left) with a rudimentary horn (right). With additional ultrasound, a differential diagnosis would be possible between Class 4a (with rudimentary cavity) and Class 4b (without rudimentary cavity) morphology
The benefit of direct endoscopic visualisation of the pelvis and uterine cavity be balanced against the risk of related complications. Laparoscopy is not an innocuous procedure with up to 50 % of complications related to laparoscopic entry [39].
Conclusion
Hysteroscopy and laparoscopy continue to be considered the gold standard for the identification of congenital uterine anomalies; both techniques are inconvenient, because they cannot provide information on the composition of the soft tissues, like the uterine muscular wall, or the presence of a rudimentary cavity. The currently available data provide strong evidence that the non-invasive 3-D ultrasound /3-D SIS technique is very accurate for diagnosing non-complex uterine anomalies (classes U1, U2, U3). For that purpose, the latter technique can be considered the preferred method, and it may become a mandatory procedure. The visualisation of the contours of uterine soft tissue is an added value; it allows the differential diagnosis between U2 and U3 abnormalities. In more complex cases, the full arsenal of diagnostic tools should be used, including a clinical examination, 3-D ultrasound, MRI, hysteroscopy, and laparoscopy. Direct visualisation with hysteroscopy and laparoscopy will provide information on the presence of concomitant pathology that can impair fertility. Performance of a full exploration will enable the physician to provide the patient with exact information and obtain fully informed consent before attempting a surgical correction.
References
1.
Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Hum Reprod Update. 2008;14:415–42.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


