Diagnosis of Obstructive Sleep Apnea
Introduction
Sleep-disordered breathing (SDB) is a common and serious cause of morbidity during childhood. This chapter is concerned with diagnosing the spectrum of obstructive SDB, ranging from the frank, intermittent occlusion seen in obstructive sleep apnea syndrome (OSAS), to persistent, primary snoring (PS). OSAS is characterized by recurrent episodes of partial or complete airway obstruction resulting in hypoxemia, hypercapnia, and/or respiratory arousal (Figure 28-1). The sleep fragmentation and gas exchange abnormalities observed with OSAS may produce serious cardiovascular and neurobehavioral impairment. The upper airway resistance syndrome (UARS) is characterized by brief, repetitive respiratory effort-related arousals (RERA) during sleep in the absence of overt apnea, hypopnea, or gas exchange abnormalities.1 It has been linked to significant cognitive and behavioral sequelae in children including learning disabilities, attention deficit, hyperactivity, and aggressive behavior.2 Obstructive hypoventilation (OH) features prolonged increased upper airway resistance accompanied by gas exchange abnormalities, but not frank apnea or hypopnea.3 Children with PS may have increased respiratory effort, but lack identifiable arousals, including respiratory-effort-related, EEG and subcortical arousals.4 Though PS has been traditionally defined as a benign condition, without polysomnographic abnormalities,5 recent evidence suggests that the increased respiratory effort in PS per se may be associated with untoward neurobehavioral consequences.6–9
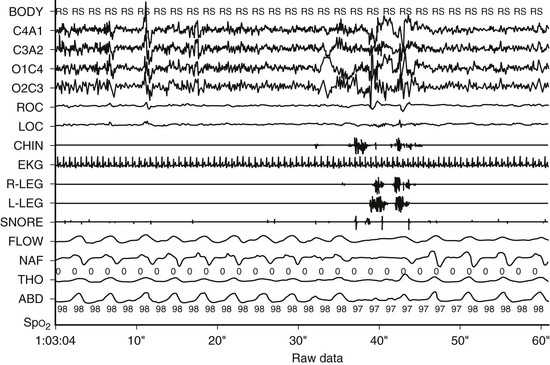
Figure 28-1 A 60-second epoch from a 16-year-old with snoring and excessive daytime sleepiness. Note flow limitation and reduction in the amplitude of the nasal pressure tracing leading to an EEG arousal. Inspiration is upward. Flow, thermistor; NAF, nasal pressure; Tho, thorax; Abd, abdomen; Body, body position; RS, right side; SpO2, pulse oximetry.
Habitual snoring has been reported in 3–12% of the general pediatric population, although only 1–3% will have OSAS.10–12 Early recognition of SDB is important insofar as treatment with adenotonsillectomy or continuous positive airway pressure is effective. Establishing strict criteria for OSAS diagnosis and severity is the basis for optimizing the surgical and medical management of this condition.13 The diagnosis and management of pediatric OSAS continues to evolve as more precise measures of flow limitation and sleep fragmentation are introduced. Both the intermittent hypoxemia and sleep fragmentation characteristic of OSAS pose a risk to the vulnerable developing brain. The increased recognition of subtle neurocognitive impairments in children with SDB has forced clinicians to rethink the threshold level of disease requiring intervention.
History and Physical Examination
The high incidence of OSAS in children mandates that screening inquiries about sleep disturbances should be a routine part of the primary care interview14 (Table 28.1). Particular attention should be given to conditions known to exacerbate SDB such as craniofacial abnormalities, neuromuscular weakness, and genetic conditions. A parent typically provides the clinical history, with the patient oblivious to their condition, other than sometimes the complaint of excessive daytime sleepiness. Nightly snoring is observed in most children with OSAS. However, snoring may be absent in the setting of craniofacial abnormalities in infants.15 A parental report of a snoring child is an accurate predictor of polysomnographic snoring, but not of OSAS.16 In addition, a population-based study using home-based polysomnography found that loud snoring one to two times per week during the last month was absent in at least 25% of children with documented OSAS.17 Snoring is often accompanied by labored breathing, hyperextension of the neck, and witnessed apneic pauses. Subjective reports of excessive daytime sleepiness are less common in young children with OSAS, although they are often present in adolescents. A recent study reported that only 7.5% of children with polysomnographically proven OSAS had a history of EDS.18 Using an objective measure of sleepiness, such as the multiple sleep latency test, reveals that only 13% of children with OSAS have a sleep latency <10 minutes.18 However, the incidence of objective sleepiness is higher in obese children with OSAS.19,20 Also, although not considered sleepy by adult standards, children with SDB may be relatively sleepier than normal children.21 Increasing BMI and apnea index (usually greater than 15–20 events/hour) have been independently correlated with shorter sleep latencies.18
Table 28.1
| SLEEP | WAKEFULNESS |
| Snoring | Poor school performance |
| Witnessed apnea | Aggressive behavior |
| Choking noises | Hyperactivity |
| Increased work of breathing | Attention deficit disorder |
| Paradoxical breathing | Excessive daytime sleepiness |
| Enuresis | Morning headaches |
| Mouth breathing | Age-inappropriate napping |
| Restless sleep | Difficult to arouse from sleep |
| Diaphoresis | Depression |
| Hyperextended neck | |
| Frequent awakenings | |
| Dry mouth |
The use of standardized screening questionnaires for OSAS has been disappointing. Brouillette et al. presented a questionnaire aimed at distinguishing children with OSAS from normal controls.22 However, subsequent application of this and other questionnaires to a population of snoring children demonstrated a wide-ranging positive predictive value, 48.3–76.9%, and negative predictive value, 26.9–93%.22–25 Although children with OSAS are statistically more likely to have reported symptoms such as witnessed apnea, cyanosis, and/or labored breathing, no questionnaire has a sufficiently high positive predictive value and negative predictive value to be used as a primary diagnostic tool.25,26 Thus, the clinical history alone is insufficient to diagnose OSAS amongst a population of snoring children. Questionnaires have also been developed that incorporate questions relating to the consequences of OSA, including hyperactivity and behavior. An affirmative response to at least one-third of these questions on the 22-question Pediatric Sleep Questionnaire had a sensitivity of 0.85 and a specificity of 0.87.27,28
Physical examination of children with OSAS is most often normal, with the exception of adenotonsillar hypertrophy or craniofacial abnormalities (Box 28-1). The majority of children with OSAS are of normal height and weight, although both obesity and failure to thrive may occur. Cardiovascular sequelae of SDB such as cor pulmonale and congestive heart failure are infrequently observed in current clinical practice, as heightened awareness has facilitated earlier diagnosis. Although blood pressure is statistically elevated in children with OSAS, the wide range of normal makes this measurement a poor screening tool.29 The neurocognitive consequences of OSAS are non-specific, such as poor school performance,30 aggressive behavior, and hyperactivity.31
Imaging and Laboratory Evaluation
The diagnosis of SDB is firmly established using polysomnography, and ancillary testing is rarely indicated. Further screening may be useful to facilitate the perioperative care and to exclude underlying conditions (Box 28-2). For example, concern regarding right ventricular dysfunction may necessitate an EKG or echocardiogram. Occasionally, chronic intermittent hypoxemia may induce polycythemia, while persistent hypercarbia can elevate serum bicarbonate. Routine pulmonary function testing is not indicated in children suspected of having OSAS unless restrictive or obstructive lung disease is suspected. A fluttering pattern in the flow volume loop has been described in adults with OSAS.32 Magnetic resonance imaging (MRI) of the upper airway in children with OSAS compared to normal controls reveals a statistically smaller upper airway luminal volume and elongation of the soft palate, as well as enlarged tonsils and adenoids.33 However, there is considerable overlap between the groups, rendering MRI a poor screening tool. The measurement of the ratio of the width of the tonsil to the depth of the pharyngeal space on a lateral neck radiograph was reported to have a good sensitivity and specificity for distinguishing mild from moderate/severe OSAS in a small number of patients.34 Adenoidal enlargement measured by cephalometry was present in over 80% of children with OSAS but also in 42% with primary snoring.35 Though neck radiographs may be suggestive of adenoidal hypertrophy, direct visualization of the adenoids remains the diagnostic standard.36 Dynamic fluoroscopy under sedation may demonstrate glossoptosis, particularly in children with macroglossia, micrognathia, or neuromuscular weakness.37 Anatomical localization of the site of obstruction with cine MRI may alter the therapeutic approach in some children with residual OSAS following adenotonsillectomy or craniofacial anomalies.38 Other promising but understudied tools for identifying OSAS in children include nasal rhinometry39 and acoustic pharyngometry.40
Video/Audio Recordings
Diagnosing OSAS using home audio recordings, in addition to a standard clinical history and physical examination, revealed a sensitivity of 71–92% and a specificity of 29–80%.41,42 Subtle forms of SDB are particularly difficult to evaluate using this technique. However, computer-aided processing of audio signals for regularity may improve predictive value.43 Frequency domain analysis of the snoring signal has also shown promise in distinguishing OSAS from PS.44 Video recordings can also yield a noninvasive measure of movement and therefore arousal.45–47 Video is also a useful adjunct to a comprehensive polysomnogram to evaluate body and head positioning, paradoxing, snoring, and mouth breathing. Studies correlating video scoring systems to standard polysomnography have been encouraging.47 Future research will be necessary to validate the utility of a particular domiciliary video/audio study in a population with a well-characterized symptomatology.
Overnight Polysomnography
Polysomnography represents the gold standard for establishing the presence and severity of SDB in children, and can be performed in children of all ages. An expert consensus panel from the American Academy of Pediatrics has recommended overnight polysomnography as the diagnostic test of choice in evaluating children with suspected SBD.14 Guidelines for performing laboratory-based polysomnography in children have been established.48,49 The sleep laboratory should be a non-threatening environment that comfortably accommodates a parent during the study. Personnel with pediatric training should record, score, and interpret the study. The use of sedatives50 and sleep deprivation51 may worsen SDB, and is therefore not recommended. To the extent possible, sleep studies should conform to the child’s usual sleep period. Infants may reasonably be studied during the day, while adolescent studies should generally start later at night. The polysomnographic montage will vary with the patient’s suspected disorder (Box 28-3).
Electroencephalogram
Consensus guidelines for analyzing sleep architecture have been established in infants,48,52,53 children,48,52 and adults.48 Standard practice is to apply adult EEG criteria to children older than 2–3 months of age. Sleep staging establishes that an adequate amount of total sleep time (TST) and sufficient REM sleep was obtained on the night of the study, and demonstrates the presence or absence of sleep fragmentation. In addition to sleep staging, the EEG tracing is useful for scoring cortical arousals and detecting epileptiform discharges. By consensus, an electrocortical (EEG) arousal is defined in adults as an abrupt 3-second shift in EEG frequency.48 These criteria appear to be appropriate for use in children as well.54 However, visible EEG arousals are present in only 51% of obstructive events in children,55 complicating the diagnosis of UARS in children. Frequency domain analysis of the EEG tracing may further enhance the sensitivity of detecting respiratory events or arousal.56,57 Initial reports indicated that children with snoring58 or even severe OSAS may have normal sleep state distribution.59 However, a large cohort (n = 559) comparing normal children to children with OSAS revealed that OSAS patients have increases in slow-wave sleep (23.5 vs. 28.8% TST) and decreases in REM sleep (22.3 vs. 17.3% TST).60 Furthermore, these authors observed a decline in the spontaneous arousal index in OSAS patients versus controls (8.4 vs. 5.3), suggesting a homeostatic elevation in the arousal threshold.60
Arousal
Arousal from sleep is a protective reflex mechanism that restores airway patency through dilator muscle activation. Both mechano- and chemoreceptors have a role in initiating the arousal response. Though arousals reverse the airway obstruction, they result in the untoward consequences of sleep fragmentation and sympathetic activation.61 Polysomnographically, arousal may be associated with EEG changes,57 increased airflow, elimination of airflow limitation, cessation of paradoxical breathing, tachycardia, movement,45 blood pressure elevation,62 and autonomic activation. In children, however, approximately 50% of obstructive events do not result in an EEG arousal.55 In infants, EEG arousals are even less common.55 Thus, depending on the EEG arousal index for the diagnosis of UARS is unreliable. Frequency domain analysis may reveal evidence of EEG arousal not readily visible, and may be a clinically useful tool in the future.56,57 Obstructive events that terminate with autonomic activation are termed subcortical arousals. Autonomic measures include heart rate variability,63 blood pressure elevations,62 pulse transit time,4 and peripheral arterial tonometry.64 The pulse transit time was reported to be a more sensitive measure of respiratory arousal compared to 3-second EEG arousals.4 As a screening tool for OSAS, the pulse transit time had a sensitivity of 81% and a specificity of 76%.65 Subcortical arousals alone have been demonstrated to result in neurocognitive impairment in adults.66
Measures of Respiratory Movements
A variety of methodologies to categorize central and obstructive respiratory events is amenable to overnight polysomnography. Thoracic and abdominal excursion is measured most commonly with respiratory inductive plethysmography (RIP). The classification of central and obstructive apneas is achieved by determining whether respiratory efforts are present during intervals of reduced flow. Uncalibrated RIP tracings may also be used as an index of thoraco-abdominal asynchrony. The highly compliant chest wall in children results in asynchronous motion between the thoracic and abdominal tracings, termed paradoxical breathing. This disparity may be quantified using phase angle analysis that is independent of the relative contribution of the two compartments.67 Thoraco-abdominal asynchrony has been demonstrated in children with increased respiratory effort due to upper airway obstruction and OSAS.67,68 Paradoxical breathing is normally seen in infants due to the high compliance of their chest wall, particularly during REM sleep, but is rare after 3 years of age.69
Esophageal manometry (Pes) represents the gold standard for quantifying respiratory effort and permits the detection of subtle, partially obstructive events that may produce sleep fragmentation.70 However, Pes monitoring is uncomfortable and may itself alter the frequency of respiratory events.71 The introduction of non-invasive, nasal pressure measurements has largely supplanted Pes in establishing the diagnosis of UARS (see Figure 28-1). Nevertheless, esophageal manometry peak amplitude and percent of time spent lower than −10 cmH2O has been reported to have a better correlation with behavioral outcomes than the traditional apnea–hypopnea index with or without respiratory-effort-related arousals.72 Current practice is to reserve Pes monitoring for rare cases of children with diagnostic uncertainty even after standard overnight polysomnography.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


