GENERAL CONSIDERATIONS
Type 1 diabetes (T1D) is the most common type of diabetes mellitus in people younger than 20 years, but can develop at any age and most cases are diagnosed after age 20. The classical presentation includes increased thirst (polydipsia), urination (polyuria), and weight loss; however, the patient may be overweight or even obese. T1D is further divided into T1a (autoimmune) (~95% of the cases) and T1b (idiopathic) diabetes. T1a diabetes is marked by presence of autoantibodies to islet cell autoantigens (insulin, GAD65, IA-2, and ZnT8) and high-risk HLA (human leucocyte antigen) haplotypes (DR4, DQ8, and DR3/DQ2). Insulin production, measured by fasting or stimulated C-peptide levels, is usually low. In the United States, T1D affects an estimated 1.5 million people, including 160,000–200,000 patients younger than age 20 (~25,000 diagnosed annually).
Type 2 diabetes (T2D) is a heterogeneous phenotype diagnosed most often in persons older than age 40 who are usually obese and initially not insulin-dependent. T2D is rare before age 10, but has increased in frequency in older children as a consequence of the epidemic of obesity. The vast majority of the 26 million patients with diabetes in the United States have T2D, but only approximately 16,000 patients are younger than age 20 (~5000 diagnosed annually).
Monogenic forms of diabetes can be diagnosed at any age. They account for less than 1% of childhood diabetes, but form the majority of cases diagnosed before the ninth month of life. Neonatal diabetes is transient in about half of the cases; if persistent, it presents a significant clinical challenge. Some infants respond better to sulfonylurea than insulin. Maturity-onset diabetes of the young (MODY) presents as a nonketotic and usually non–insulin-dependent diabetes in the absence of obesity or islet autoantibodies. A strong family history of early-onset diabetes is common. The most frequent forms are due to mutations in glucokinase or hepatic nuclear factor 1 or 2 genes. Glucokinase mutations rarely require therapy; other forms respond to oral hypoglycemic agents or insulin. Commercial and research-oriented genotyping services are available to aid correct diagnosis.
 Pathogenesis
Pathogenesis
A. Type 1 Diabetes
Type 1 diabetes results from autoimmune destruction of the insulin-producing β cells of the pancreatic islets. This destruction occurs over months or years and symptoms do not appear until most of the pancreatic islets have been destroyed.
The incidence is the highest in children of European ancestry, followed by African Americans and Hispanics; the rates are low in Asians and Native Americans.
About 6% of siblings or offspring of persons with T1D also develop diabetes (compared with prevalence in the general population of 0.2%–0.3%). However, fewer than 10% of children newly diagnosed with T1D have a parent or sibling with the disease. More than 90% of children with T1D carry at least one of the two high-risk HLA haplotypes—DR4/DQ8 or DR3/DQ2—and 40% of US children diagnosed before age of 10 years have both (one from each parent), compared with only 2.5% of the general population. Over 30 non-HLA genetic variants have also been implicated.
Since the 1950s, the incidence of T1D has increased dramatically worldwide, doubling approximately every 20 years. Despite much research of early childhood infections and diet, the environmental factor(s) responsible for this epidemic are poorly defined. There is no effective prevention as of 2013; however, screening of high-risk groups for islet autoantibodies and intensive follow-up reduces the severity of the presentation.
B. Type 2 Diabetes
Type 2 diabetes has a strong genetic component, although the inherited defects vary in different families. Obesity, particularly central, and lack of exercise are major causes, but rarely sufficient alone to cause diabetes in youth. Most pediatric patients come from low socioeconomic strata and dysfunctional families; some present with psychiatric disorders. Obesity, T2D, and associated insulin resistance adversely affect cardiovascular health. Acanthosis nigricans, a thickening and darkening of the skin over the posterior neck, armpits, or elbows, is present in many obese children and has occasionally contributed to the diagnosis of T2D.
 Prevention
Prevention
A. Type 1 Diabetes
Islet autoantibodies are present for months to years prior to diagnosis in the serum of patients who develop T1a diabetes. Free antibody screening is now available for families having a relative with type 1 diabetes (1-800-425-8361). These antibodies, which do not mediate β-cell destruction, offer a useful screening tool. The β-cell damage is mediated by T lymphocytes. Immunosuppression at different checkpoints of the autoimmune process can slow down the damage, but has no durable effect when stopped. Immunomodulation, including induction of tolerance to islet autoantigens, with or without immunosuppression, is an area of intensive research.
B. Type 2 Diabetes
The Diabetes Prevention Program in adults with impaired glucose tolerance found that 30 minutes of exercise per day (5 d/wk) and a low-fat diet reduced the risk of diabetes by 58%. Taking metformin also reduced the risk of T2D by 31%.
 Clinical Findings
Clinical Findings
A combination of polyuria, polydipsia, and weight loss in a child is unique to diabetes. Unfortunately, these symptoms are often missed by primary care providers or even emergency department staff. The frequency of diabetic ketoacidosis (DKA) in US children has not decreased in the past 20 years and is approaching 40%, a sign of poor provider and poor community awareness. More than half of DKA patients were seen by a provider in days preceding diagnosis and obvious symptoms and signs were missed. In contrast, only 10%–20% of newly diagnosed children in Scandinavia or Canada present with DKA. This embarrassing statistic could be greatly improved with better history taking and point-of-care urine analysis. Initial diagnosis can be easily confirmed by blood glucose and ketones measurement using widely available and inexpensive meters.
The clinical presentation of DKA includes abdominal pain, nausea, and vomiting that can mimic an acute abdomen. The patients are mildly to moderately dehydrated (5%–10%), may have Kussmaul respiration, and become progressively somnolent and obtunded. The distribution of diagnosis has shifted to younger age; infants, toddlers, and preschool age children are at particular risk. They often have symptoms of minor infection or gastrointestinal upset. A heavy diaper in a dehydrated child without diarrhea should always flash an alarm. Blood or urine glucose levels could be lifesaving. Blood glucose higher than 200 mg/dL in a child is always abnormal and must be promptly and meticulously followed in consultation with a pediatric endocrinology service. If the presentation is mild and an outpatient diabetes education service is available, hospitalization is usually not necessary.
Transient, “stress-” or steroid-induced hyperglycemia can occur with illness. In a well child, the diagnosis must not be based on a single plasma glucose test or a borderline result obtained using a glucose meter. Our center routinely tests such children for islet autoantibodies to rule out ongoing islet autoimmunity. Absence of the three most available autoantibodies (to insulin, GAD, and IA-2) provides 80% negative predictive value. If HbA1c is normal, we recommend home monitoring of blood glucose for several days. In children progressing to overt diabetes, hyperglycemia after dinner is usually the initial abnormality, which is detectable by self–blood glucose monitoring at home.
In the presence of typical symptoms, a random blood glucose level above 200 mg/dL (11 mmol/L) (confirmed on a CLIA [Clinical Laboratory Improvement Amendments]-certified instrument) is sufficient to make the diagnosis of diabetes. An oral glucose tolerance test is rarely necessary in children. In borderline or asymptomatic cases, a fasting plasma glucose level over 126 mg/dL (7 mmol/L) or a plasma glucose level above 200 mg/dL (11.1 mmol/L) 2 hours after an oral glucose load (1.75 g glucose/kg up to a maximum of 75 g) on 2 separate days confirms the diagnosis. Impaired (not yet diabetic) fasting glucose values are 100–125 mg/dL (5.5–6.9 mmol/L) and impaired 2-hour values are 140–200 mg/dL(7.8–11.1 mmol/L). Children with impaired fasting glucose or impaired glucose tolerance are at high risk of T2D and require careful follow-up and lifestyle modification with weight loss, if obese.
 Treatment
Treatment
Major variables in T1D treatment are insulin therapy, diet, exercise, and psychosocial support. All must be addressed to achieve safe and effective metabolic control. At present, the safest recommendation for glycemic control in children is to achieve an HbA1c < 7.5% or the lowest that can be sustained without severe hypoglycemia or frequent moderate hypoglycemia. The HbA1c level reflects the average blood glucose levels over the previous 3 months. Each child should have targets individually determined.
Intensive diabetes management includes: (1) three or more insulin injections per day, or insulin pump therapy based on carbohydrate counting; (2) at least four blood glucose determinations per day; (3) ketone monitoring during hyperglycemia; and (4) frequent contact with a diabetes healthcare provider. The Diabetes Control and Complications Trial (DCCT) showed that this approach improved HbA1c to approximately 7% and significantly reduced the risk for retinal, renal, cardiovascular, and neurologic complications of diabetes.
A. Patient and Family Education
Education about diabetes for all family members is essential for the home management of diabetes. The use of an educational book (see Understanding Diabetes and Understanding Insulin Pumps and Continuous Glucose Monitors) can be very helpful to the family. All caregivers need to learn about diabetes, how to give insulin injections, perform home blood glucose monitoring, and handle acute complications. Although teenagers can be taught to perform many of the tasks of diabetes management, they do better when supportive, not overbearing, parents continue to be involved in management of their disease. Children younger than age 12 years cannot reliably administer insulin without adult supervision because they may lack fine motor control and/or may not understand the importance of accurate dosage.
B. Insulin
Insulin has three key functions: (1) it allows glucose to pass into the cell for oxidative utilization; (2) it decreases the physiologic production of glucose, particularly in the liver; and (3) it turns off lipolysis and ketone production.
1. Treatment of new-onset diabetes—Children presenting in DKA (pH < 7.30 or bicarbonate < 15 mEq/L in presence of hyperglycemia and ketosis/ketonemia) require intravenous insulin in addition to replacement of fluids and electrolytes. Regular insulin is given as a continuous drip at a rate of 0.05–0.1 U/kg/h to achieve drop in blood glucose of approximately 100 mg/dL per hour. Giving an IV insulin bolus of insulin before the completion of the initial fluid bolus has been associated with brain edema. Fast-acting insulin analogs have no advantage over regular insulin when given intravenously.
In children who present without DKA and have adequate oral intake, the initial insulin dose can be administered subcutaneously as 0.2 U/kg of short-acting insulin (regular) or, preferentially, rapid-acting analog: lispro (Humalog), aspart (NovoLog), or glulisine (Apidra). At the same time, 0.2–0.3 U/kg of long-acting insulin analog—glargine (Lantus) or detemir (Levemir)—can be administered subcutaneously to limit the need for multiple insulin injections. This usually suffices for the initial 12–24 hours preceding systematic diabetes education.
The dose is adjusted with each injection during the first week. The rule of thumb is to start insulin at the low end of the estimated daily requirement and titrate it up based on frequent (q2–4h) blood-glucose monitoring. The initial daily dose of insulin is higher in the presence of ketosis, infection, obesity, or steroid treatment. It also varies with age and severity of onset. A total subcutaneous daily dose of 0.3–0.7 U/kg/day may suffice in prepubertal children, while pubertal or overweight children and those with initial HbA1c > 12% commonly require 1.0–1.5 U/kg/day of insulin during the initial week of treatment.
The insulin dose peaks about 1 week after diagnosis and decreases slightly with the waning of glucotoxicity and voracious appetite. Approximately 3–6 weeks after diagnosis, most school-children and adolescents experience a partial remission or “honeymoon period.” Temporary decrease in the insulin dose during this period is necessary to avoid severe hypoglycemia. The remission tends to last longer in older children, but is rarely complete and never permanent. Other types of diabetes should be considered in patients with unusually low insulin requirements.
2. Long-term insulin dosage—Children usually receive a rapid-acting insulin to cover food intake or correct high blood glucose and a long-acting insulin to suppress endogenous hepatic glucose production. This is achieved by combining insulins with the desired properties. Understanding the onset, peak, and duration of insulin activity is essential (Table 35–1).
Table 35–1. Kinetics of insulin action.
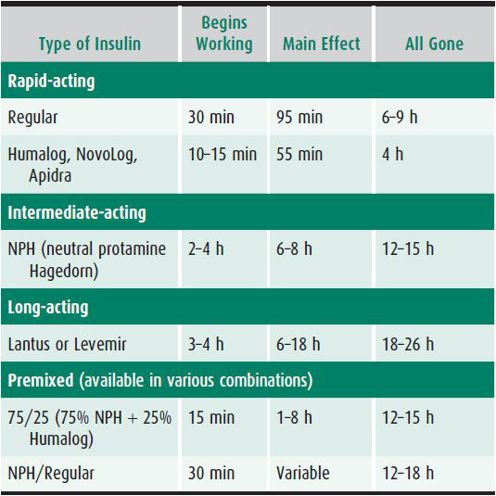
Nearly all children diagnosed with T1D at our center receive a basal-bolus multiple daily injection (MDI) treatment. This usually consists of 3–4 injections (boluses) of rapid-acting analog before meals and 1–2 injections of long-acting analog insulin. The dose of premeal rapid-acting insulin is calculated based on anticipated carbohydrate content of the meal and additional insulin to correct for high blood glucose, if needed. Sliding scales for dosing of rapid-acting insulin are helpful initially, while families learn carbohydrate counting. This shortcut assumes that the content of carbohydrates does not vary, for example in dinner, from day to day, this may lead to significant under- and overdosing.
Children younger than 4 years usually need 1 or 2 units of rapid-acting insulin to cover carbohydrate intake. Children aged 4–10 years may require up to 4 units of rapid-acting insulin to cover breakfast and dinner, whereas 4–10 units of rapid-acting insulin are used in older children. These estimates do not include correction for high blood glucose.
Families gradually learn to make small weekly adjustments in insulin dosage based on home blood glucose testing. Rapid-acting analog insulin is given 10–20 minutes before eating to account for delay in insulin action. If slower human regular insulin is used, the injections should be given 30–60 minutes before meals—rarely a practical option. In young children who eat unpredictably, it is often necessary to wait until after the meal to decide on the appropriate dose of rapid-acting insulin, which is a compromise between avoiding hypoglycemia and tolerating hyperglycemia after meals.
A long-acting analog insulin glargine (Lantus) or detemir (Levemir) is given once or twice a day to maintain basal insulin levels between meals. Daily adjustments in long-acting insulin dose usually are not needed. However, decreases should be made for heavy activity (eg, sports, hikes, or overnight events).
In the past, most children would receive 2 injections per day of a rapid-acting insulin and an intermediate-acting insulin (NPH), often mixed just before injection. About two-thirds of the total dosage would be given before breakfast and the remainder before dinner. This regiment has been shown to be inferior in achieving recommended HbA1c levels and avoiding hypoglycemia, compared with the basal-bolus regiment described above. Analog insulin (glargine, detemir) works more efficiently than NPH. When changing a patient from NPH insulin to an analog, initially only 50% of the daily units of long-acting insulin is recommended.
3. Insulin pump treatment—Continuous subcutaneous insulin (insulin pump) therapy is the best way to restore the body’s physiologic insulin profile. The pump delivers a variable programmed basal rate that corresponds to the diurnal variation in insulin needs. Prepubertal children require higher basal rate in the early part of night, while postpubertal patients who experience the “dawn phenomenon” require higher rates in the morning. The user initiates bolus doses before meals and to correct hyperglycemia. Most pumps can receive wireless transmission of test results from glucose meters, but the patient or caregiver must still manually enter the amount of carbohydrate being consumed. The pump calculates the amount of insulin needed for a meal or correction based on previously entered parameters which include: insulin-to-carbohydrate ratios, insulin sensitivity factor, glycemic target, and duration of insulin action (set at 3–4 hours to protect from accumulating too much insulin). The user may override the suggestion or press a button to initiate the bolus.
Most clinical trials have demonstrated better HbA1c and less severe hypoglycemia with pump therapy, compared to MDI. Pump therapy can improve the quality of life in children who have trouble with or fear of injections or who desire greater flexibility in their lifestyle; for example, with sleeping in, sports, or irregular eating. Insulin pumps can be particularly helpful in young children or infants who have multiple meals and snacks and require multiple small doses of rapid-acting insulin. The newer generation of insulin pumps can deliver as little as 0.025 U/h, but higher rates using diluted insulin may be needed for uninterrupted flow.
Compliance problems include infrequent blood glucose testing, not reacting to elevated blood glucose, incorrect carbohydrate counting, or missing the boluses altogether. Side effects of insulin pump treatment include failures of insulin delivery because of a displaced or obstructed infusion set. Insulin pump treatment is significantly more expensive than regimens based on injections. For some patients, pumps may be too difficult to operate, or they cannot comply with the multiple testing and carbohydrate counting requirements, or the pump is unacceptable because of body image issues or extreme physical activity (swimming, contact sports). Disposable pumps are already available and much smaller “patch” pumps are in development.
4. “Closed-loop” systems—In the near future, insulin pumps will be directed automatically by a continuous glucose sensor (see section Home Blood Glucose Measurements) with minimal human input. Simple systems are already available that feature, for instance, sensor-initiated automatic suspension of insulin delivery at a predetermined low-glucose level and automatic resumption of the delivery at a safe level. Others, controlling postprandial glycemia are in clinical trials. A number of issues remain to be solved, for example, the accuracy and biocompatibility of the sensors and infusion sets, limitations (lag time) of the systemic versus intraportal administration of insulin, lack of counterbalancing delivery of glucagon, and optimal delivery algorithms for various meals and activities.
5. Treatment of type 2 diabetes—Treatment of type 2 diabetes in children varies with the severity of the disease. If the HbA1c is still near normal, modification of lifestyle (preferably for the entire family) is the first line of therapy. This must include reducing caloric intake and increasing exercise. With mildly elevated HbA1c (6.2%–8.0%) and no ketosis, metformin is usually started at a dose of 500 mg twice daily along with modification of lifestyle. If needed, and if gastrointestinal adaptation has occurred, the dose can be gradually increased to 1 g twice daily. If the presentation is more severe, with ketosis, the initial treatment is similar to that of T1D, including IV or subcutaneous insulin. Of note, 10% of children with T2D present in DKA. Oral hypoglycemic agents may be tried at a later date, particularly if weight loss has been successful.
C. Diet
A thorough dietary history should be obtained including the family’s dietary habits and traditions, the child’s typical meal times, and patterns of food intake. Nutritional management in children with diabetes does not require a restrictive diet, just a healthy dietary regimen that the children and their families can benefit from. Insulin pump and MDI therapy utilize carbohydrate counting in which the grams of carbohydrate to be eaten are counted and a matching dose of insulin is administered. This plan allows for the most freedom and flexibility in food choices, but it requires expert education and commitment and may not be suitable for many families or situations, such as for school lunches and teenagers. Alternatively to a precise carb counting, “exchanges” are taught to estimate 10- or 15-g servings of carbohydrate.
A constant carbohydrate meal plan was used often in the past with insulin regimens based on NPH and regular insulin, where carbohydrate intake and the amount of insulin were kept relatively constant from day to day. This is now perceived as too restrictive and a potential source of conflict that will lead to poor control.
D. Exercise
Regular aerobic exercise is important for children with diabetes. Exercise fosters a sense of well-being; helps increase insulin sensitivity (a drop in glycemia in response to insulin); and helps maintain proper weight, blood pressure, and HDL-cholesterol levels.
Hypoglycemia during exercise or in the 2–12 hours after exercise can be prevented by careful monitoring of blood glucose before, during, and after exercise; reducing the dosage of the insulin active at the time of (or after) the exercise; and by providing extra snacks. Fifteen grams of glucose usually covers about 30 minutes of exercise. The use of drinks containing 5%–10% dextrose, such as Gatorade, during the period of exercise is often beneficial. Insulin dose for meals as well as the basal insulin pump rate should be reduced before, during, and sometimes after the exercise; the longer and more vigorous the activity, the greater the reduction in insulin dose.
E. Psychological Care
The diagnosis of T1D changes lives of the affected families and brings on relentless challenges. It is impossible to take “vacation” from diabetes without some unpleasant consequences. The stress imposed on the family around the time of initial diagnosis may lead to feelings of shock, denial, sadness, anger, fear, and guilt. Meeting with a counselor to express these feelings at the time of diagnosis helps with long-term adaptation. Children with T1D and their parents often experience difficult adjustment. Persisting adjustment problems may indicate underlying dysfunction of the family or psychopathology of the child or caregiver. Young people with T1D are more frequently diagnosed with and treated for psychiatric disorders, disordered eating, neurocognitive, learning problems, and poor coping skills than the general population.
Routine assessment should be made of developmental adjustment to and understanding of diabetes management, including diabetes-related knowledge, insulin adjustment skills, goal setting, problem-solving abilities, regimen adherence, and self-care autonomy and competence. This is especially important during late childhood and prior to adolescence. General and diabetes-related family functioning such as communication, parental involvement and support, and roles and responsibilities for self-care behaviors need to be assessed. Teaching parents effective behavior management skills, especially at diagnosis and prior to adolescence, emphasizes involvement and support, effective problem-solving, self-management skills, and realistic expectations. Adolescents should be encouraged to assume increased responsibility for diabetes management, but with continued, mutually agreed parental involvement and support. The transition to adult diabetes care should be negotiated and planned between adolescents, their parents, and the diabetes team well in advance of the actual transfer.
F. Home Blood Glucose Measurements
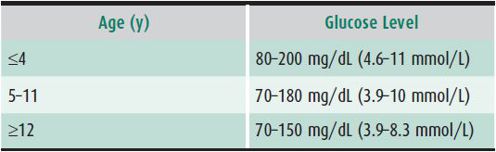
All families must be able to monitor blood glucose levels at least four times daily—and more frequently in patients who have glucose-control problems or intercurrent illnesses. Blood glucose levels can be monitored using any downloadable meter. Target levels when no food has been eaten for 2 or more hours vary according to age (Table 35–2). At least half of the values must be below the upper limit to have a good HbA1c.
Table 35–2. Ideal glucose levels after 2 or more hours of fasting.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





